2. Karamay Vocational and Technical College, Karemay 834000, China;
3. Daqing Research Institute of Applied Technology, Daqing 163316, China
2. 克拉玛依职业技术学院, 新疆 克拉玛依 834000;
3. 大庆应用技术研究院, 黑龙江 大庆 163316
Light olefins, are very important feedstocks in modern petroleum and chemical industry. Especially propylene, for example, are exclusively important raw materials for propylene oxide, polypropene, acrylic acid and other derivatives[1]. Currently, the need of propylene is increasing continuously. However, the conventional technique to get propylene cannot fully satisfy the current demand. Some new methods using other raw materials as the feed are being developed, sach as the dehydrogenation of propylene, conversion of methanol or dimethyl ether to propylene, and butene cracking[2-4]. The technology for propylene production by 1-butene cracking are drawing our attention, because the numerous and stable supply of butene from FCC and stream cracking processes.
With the deepening reseach of C4 olefin cracking prcess, more applications of this tecnique have deve-loped, sach as Propylur, Superflex, MOI, OCC etc.[5-7] Currently, ZSM-5(MFI) is widely applied to production of propylene in the 1-butene catalytic crac-king reaction[4, 8]. The hydrogen transfer reaction and aromatization reaction on the ZSM-5 lead to the increase of alkanes and C5+ hydrocarbon, and decrease propylene yield, which is the most valuable production. So, finding a new and more effective catalyst for 1-butene catalytic cracking reaction becomes the focus of this thesis. It is noteworthy that eight-membered ring microporous SAPO-18(AEI) and SAPO-34(CHA) molecular sieves revealed satisfactory performance in methanol-to-olefins (MTO)[9-10]. Some researchers once used SAPO-34(CHA) as cracking catalyst have achieved the high propylene selectivity[11-12]. Our laboratory have synthesized co-crystalline SAPO-18/SAPO-34 molecular sieves, it is applied to the catalytic cracking of 1-butene. Experimental results show that the molecular sieve structure had a critical role in the process of 1-butene catalytic cracking[13]. SAPO-18(AEI) and SAPO-34(CHA) have similar structure, we do the further reasearch of SAPO-18 molecular sieves. In this work, SAPO-18 samples of different acidity were obtained. Meanwhile, 1-butene cracking was carried out using ZSM-5, Beta and SAPO-18 with defferent ratios of Si/Al2 to research the effects of the pore demension and channel structure on the propylene selectivity and influence of acidity on the 1-butene cracking reaction.
1 Experimental 1.1 Catalyst preparationZeolites ZSM-5 and Beta were obtained from Beijing Institute of petrochemical. The Si/Al2 ratios of ZSM-5 followed by 88, 104, 163 were denoted ZSM-5-1, ZSM-5-2, ZSM-5-3 and the Si/Al2 ratios of Beta followed by 34, 55, 84 denoted Beta-1, Beta-2, Beta-3. In order to remove organic template, the ZSM-5 and Beta were calcined at 550 ℃ for 6 h under static atmosphere. Followed by ion exchange with a 0.8 mol/L ammonium nitrate solution at 70 ℃ for three times. After the ion exchange, the samples were washed with distilled water. The distilled water was dried by vacuum pump. All the above zeolites were calcined at 550 ℃ for 6 h again to obtain the H-zeolites.
SAPO-18 was synthesized following the method by Chen et al.[14]. Molecular sieve was prepared by hydrothermal synthesized from a mixture of colloidal silica and sodium aluminate, phosphate and distilled water. The initial mixture had the mole composition x SiO2:0.80 Al2O3:0.90 P2O5:50 H2O:1.60 R (x = 0.8, 0.6, 0.4), and the R is N, N-diisopropylethylamine hydroxide as template, was sealed in a Taflon-lined stainless steel autoclave and crystallized at 160 ℃ under atmospheric pressure for 8 days without agitation. After cooling the room temperature, this product was recovered by centrifugation and washed with distilled water, and then dried overnight at room temperature, and finally calcined for 5 h at 550 ℃. The morphology of sample SAPO with x =0.8, 0.6, 0.4 were denoted SAPO-18-0.8, SAPO-18-0.6, SAPO-18-0.4.
1.2 Catalyst characterizationThe sample crystallinity and phase purity were checked by X-ray diffraction patterns (XRD, Cu-Kα radiation, operating at 40 kV and 30 mA, Shimadzu, XRD-6000). XRD data were collected for 2θ between 5° and 60°. The molecular sieves crystal morphology and crystal size were obtained by scanning electron microscopy (SEM, JSM-6380). Measurement of the ca-talysts acidity by means of NH3 temperature programmed desorption (NH3-TPD) was carried out in an Auto Chem 3000 system, using a Micromeritics ASAP 2020, and the acid properties could be calculated. Measurement of specific surface area by N2 adsorption-desorption isotherms was measured on BeiShiDe 3H-2000PM1 analyzer at 77 K. The total remaining carbon was performed on a NETZSCH TG 209 F3 analyzer with the temperature-programmedrate of 20 ℃/min from 30 to 850 ℃ under oxygen flow.
1.3 Catalytic Activity EvaluationCatalytic activity tests were performed under atmospheric pressure in a continuous fixed-bed reactor. Generally, 1.0 g sample was filled in a stainless steel fixed bed reactor, which was placed in a furnace. The catalyst was heated to 500 ℃ under flowing N2 (27.3 mL/min). Thereafter, the 1-butene (≥99%) WHSV= 3.5 h-1was added in the reactor. The product distribution of samples collected at 3, 5, 10, 15 and 20 min was analyzed respectively by gas chromatography (GC-14) with a flame ionization detector (FID).
2 Result and Discussion 2.1 Characterization of molecular sieve SAPO-18In the XRD patterns is presented in Fig. 1, the peaks at 21.3°, 25.7°, 27.8° showed typical powder diffraction patterns of AEI structure and matched with those reported previously[15]. The SEM image of SAPO-18-0.8 exhibit uniform and regular cubic crystals with an average particle size of 0.5 ~ 1 μm and present high crystallinity. These are patterns show that the intrinsic AEI structure of SAPO-18 is preserved and the relative crystallinity changed only slightly. Thus, the change of Si content in the gel mixture had no obvious impact on the crystal structure of SAPO-18.
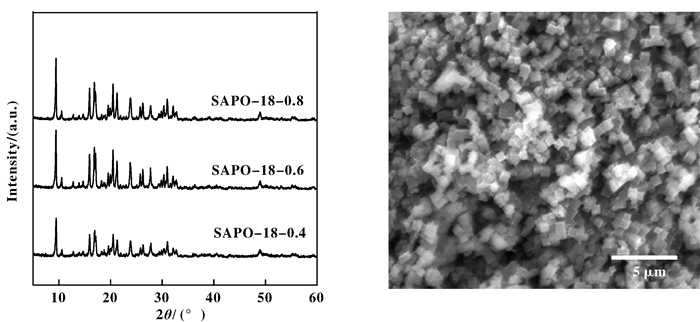
|
Figure 1 XRD patterns for SAPO-18-0.8, SAPO-18-0.6, SAPO-18-0.4 and SEM image of SAPO-18-0.8 |
Table 1 represent measurement results of NH3-TPD. There are two NH3-desorption peaks appearing at approximately 207~448 ℃ of all samples, which associate with weak and strong acid sites. However, there has been an obvious distinction between the acid strength and quantity. For example, Tmax of ZSM-5, one of which is centered in 262, 267 and 287 ℃, while the other in 434, 442 and 448 ℃, corresponding to the weak acid sites and the strong acid sites, respectively. Tmax of Beta weak acid sites centered in 258, 263, 270 ℃ and Tmax of Beta strong acid sites in 412, 420 and 423 ℃. Tmax of SAPO-18 centered in 207, 215, 220 and 396, 398, 405 ℃; corresponding to weak and strong acid sites, respectively. Hence, it is reasonable to say that the acidic strength of ZSM-5 is significantly higher than the others, while the SAPO-18 has the weakest acidic strength. On the other hand, the acidic quantity of ZSM-5 and Beta zeolites decreases with the increase of silica alumina ratio of zeolites. Beta has the highest amount of acidity (0.37~0.41 mmol/g), followed by ZSM-5 with the amount of aci-dity (0.23~0.36 mmol/g). Compared with above these zeolites, the silica content of silicoaluminophosphate molecular sieves is related to its acidic quantity intimately. Therefore, the SAPO-18 acidic quantity increases with the growth of silica content, while its amount of acidity (0.20~0.26 mmol/g) is the lowest among all samples.
| Table 1 Results of NH3-TPD and BET of various molecular sieves |
Product distribution of 1-butene catalytic cracking over different molecular sieves is listed in Table 2. The main products are ethene and propene in 1-butene ca-talytic cracking reaction in SAPO-18. Methane, propane, butanes and C5+ hydrocarbons as byproducts are also formed due to the side reactions, sach as hydrogen transfer, dehydrogenation-aromatization and dealkylation reactions.
| Table 2 Product distribution of 1-butene catalytic cracking over different molecular sieves |
Beta-1, Beta-2, Beta-3 and ZSM-5-1, ZSM-5-2, ZSM-5-3 showed high conversion above 97%~86%. As can be found in Table 1 and Table 2, more acid sites and stronger acidic strength benefit to 1-butene conversion. Meanwhile, these can further produce a large amount of alkanes (e.g. propane and butane) from olefins (e.g. propylene and butene) by hydrogen transfer reaction on these zeolites. Lin and co-workers proved that high acidity led to a large amount of hydrogen transfer reaction in catalytic cracking reaction. The results are consistent with conclusion of the literatures[15]. SAPO-18 has features of lower acidity and weaker acidic strength, which cause the lower conversion than the Beta and ZSM-5 families zeolites. However, SAPO-18 showed higher propylene selectivity and decreased the hydrogen transfer reaction effectively, as shown in Table 2.
The conversion and propylene selectivity of diffe-rent types of molecular sieves are listed in Table 3, which further support our opinion that 1-butene conversion increases with the increase of the acid strength and quantity, while propylene selectivity decreases in the series of ZSM-5 and Beta. For example, 1-butene conversion is following order: ZSM-5-1 > ZSM-5-2 ≈ ZSM-5-3, and the order of propylene selectivity is ZSM-5-1 < ZSM-5-2 ≈ ZSM-5-3. Beta shows similar results in the study. But the reaction results obtain from these samples of SAPO-18 are close to each ot-her. We suggest that these results are probably related to the similar number of acid sites in SAPO-18 framework. We can find that reaction results on Beta and SAPO-18 change significantly with the increasing of TOS, because the hydrogen transfer reaction can be suppressed by decrease acid sites. In addition, the total amount of carbon deposition with different structure is following order: SAPO-18 ≈ Beta > ZSM-5.
| Table 3 The catalytic performance comparison of different structures and acidity |
Besides acidity, another important feature of molecular sieves is the ability to act as shape selectivity catalyst for reactant, products and intermediate product owing to their pore dimension and shape of channels. The change of propylene selectivity with 1-butene conversion is showed in Fig. 2. Most of data in Fig. 2 come from the Table 3, in order to display shape selectivity catalysis of molecular sieves, the ZSM-5 catalytic cracking experiment has been added in the case of using 1-butene 82.5 h-1 WHSV as the feed and activation of the catalyst at 500 ℃ in order to obtain propy-lene selectivity under lower 1-butene conversion.
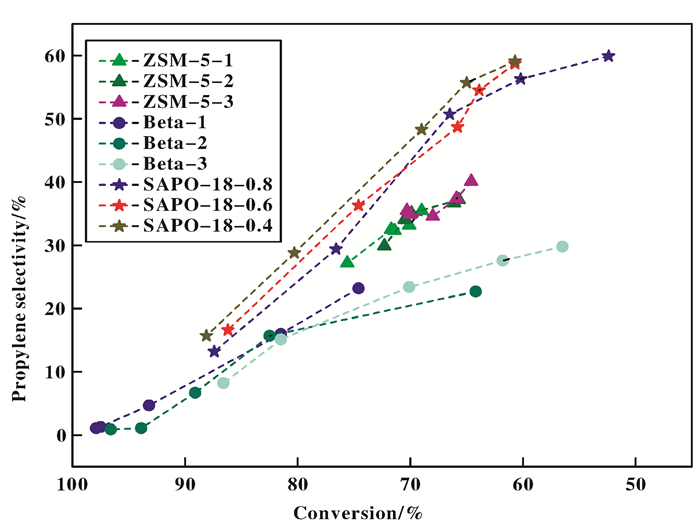
|
Figure 2 Conversion and propylene selectivity on molecular sieves with different construction and acidity |
Firstly, all three molecular sieves share a similar change trend, the propylene selectivity increases with the decrease of 1-butene conversion. This conclusion in accordance with what we discussed previously. Se-condly, improving acid sites promote the 1-butene conversion, but also increase side reaction cause propy-lene selectivity is decreased. As far as the same structure of molecular sieves is concerned, it is evidently observe that change of propylene selectivity with 1-butene conversion limits within a specific area is shown in Fig. 2. The specific area has little change as different acidity of samples with the same framework. This means that the molecular sieves of channel structure paly significant role in propylene production by 1-butene catalytic cracking reaction. Under the same 1-butene conversion condition, the propylene selectivity gradually increases according to the order of Beta, ZSM-5, SAPO-18. For example, when the conversion reached 70.0%, propylene selectivity on Beta-1, ZSM-5-2, SAPO-18-0.8 was 23.2%, 34.1%, 47.3% respectively. Selectivity of propylene can even approach 59.1% under the condition of 60.7% 1-butene conversion on SAPO-18-0.8.
ZSM-5(MFI) framework contains two overlapping channel systems, which consists of straight channels running parallel to[010] direction having 10-ring of ca. 0.51 × 0.56 nm free diameter and sinusoidal channels running parallel to [100] directions having 10-ring openings of ca. 0.51 × 0.54 nm[16]. This structure, with a high connectivity and without cavities, favors the diffusion of the aromatics. So ZSM-5 has a large number of C5+ hydrocarbons in the product distribution and the fewest weight ratio of coke among all catalysts, as shown in Table 3. ZSM-5 exhibited desirable stability under the same reaction condition. The Beta (*BEA) has one-dimensional channel structure of <100>direction 0.66 × 0.67 nm and [001] direction 0.56 × 0.56 nm[17]. Though the Beta has larger pore dimensions with 12-ring windows than ZSM-5, it deactivate faster than ZSM-5 due to absence of multidimensional channel, and the coke result is also showed in Table 3.
The SAPO-18(AEI) framework structure consists of three-dimensional eight-ring channel with pore-openging dimensions of [010] direction 0.38 × 0.38 nm [010] direction 0.38 × 0.38 nm [001] direction 0.38 × 0.38 nm and pear-shaped cage with cage dimension of 1.27 × 1.16 nm[18]. Extensive research indicated that octyl carbocations could be produced by the dimerization of butenes, which is a crucial step of 1-butene catalytic cracking reaction[15, 19-20]. In contrast to ZSM-5 and Beta, the SAPO-18 has better 1-butene catalytic cracking performance, because the SAPO-18 with pear-shaped cavity can provide more effective space for dimerization of butene and subsequent isomerization bring specific octyl carbocations to improve propylene selectivity, as listed in Scheme 1. SAPO-18 and SAPO-34 have similar cavity structure and the volume of cavity to match that of the specific octyl carbocations[18, 21], which must be effective to provide access of octyl carbocations to the active sites in wall of cavity can occur the β-scission of specific octyl carbocations generate propylene. For example the β-scission of the octyl carbocations a, b, c, d, e by closely fitting the carbocation volume to that of the molecular sieve cavity. Therefore, the SAPO-18 has remarkable shape-selective feature of intermediate products. In addition, eight-ring pore of SAPO-18 can limit macromolecular product outside the cage to further improve propylene selectivity, but it also causes inactive easily. However, the used catalyst can be quickly regenerated by fluid-bed in industry to resolve the deactivation, SAPO-18 molecular sieve with high propylene selectivity has promising development prospect in 1-butene cracking.

|
Scheme 1 The formation of specific octyl carbocations for the production of C3H6 in SAPO-18 cavity |
SAPO-18 with different contents of Si were successfully synthesized. The high acid amount improved the 1-butene conversion but led to the decrease of the selectivities of propylene owing to the side reactions (such as hydrogen transfer) derived from additional acid sites. What is more, the pore dimension and channel structure determined the available selectivity of propylene. The aromatization reaction which is easy to occur on overlapping 10-ring channel of ZSM-5 leads to the increase of C5+ hydrocarbons and the decrease of propylene selectivity. The pear-like cage of SAPO-18 with dimension of 1.27 × 1.16 nm will be more advantageous to for dimerization of 1-butene and β-scission of octyl carbocations to improve propylene selectivity. The SAPO-18 has remarkable shape-selective feature of intermediate products.
Acknowledgements: The authors would like to acknowledge the support for this work from the project of the PetroChina Innovation Foundation, China (Grant No.2012D-5006-0403), PetroChina Technology Development (2014A-2610).| [1] | Fukudome K, Suzuki T. Highly selective oxidative dehydrogenation of propane to propylene over VOx-SiO2, catalysts[J]. Catal Sur Asia, 2015, 19(3): 172–187. DOI:10.1007/s10563-015-9192-4 |
| [2] | Kogan S B, Herskowitz M. Selective propane dehydrogenation to propylene on novel bimetallic catalysts[J]. Catal Commun, 2001, 2(5): 179–185. DOI:10.1016/S1566-7367(01)00029-2 |
| [3] | Zhao T S, Takemoto T, Tsubaki N. Direct synthesis of propylene and light olefins from dimethyl ether catalyzed by modified H-ZSM-5[J]. Catal Commun, 2006, 7(9): 647–650. DOI:10.1016/j.catcom.2005.11.009 |
| [4] |
a. Zhu X, Liu S, Song Y, et al. Butene catalytic cracking to propene and ethene over potassium modified ZSM-5 catalysts[J].Catal Lett, 2005, 103 (3): 201-210. b. An Liang-cheng (安良成), Jiang Yong-jun (江永军), Wang Lin (王林), et al. High concentration synthesis of small crystal B-modified ZSM-5 zeolites and their catalytic performance for methanol to propylene reaction (高浓度体系小晶粒B改性ZSM-5分子筛的制备及甲醇制丙烯催化性能)[J]. J Mol Catal (China)(分子催化), 2016, 30 (1): 10-19. c. Guo Chun-lei (郭春垒), Wang Yin-bin (王银斌), Wang Yang (汪洋), et al. The effect of silylation on the catalytic performance of nanosize ZSM-5 zeolites for the conversion of methanol to gasoline (硅烷化改性对纳米ZSM-5甲醇制汽油催化剂性能的影响) [J]. J Mol Catal (China)(分子催化), 2016, 30 (2): 115-122. |
| [5] |
a. Bölt H V, Glanz S. Increase propylene yields cost-effectively[J]. Hydro Pro, 2002, 81 (12): 77-78, 80. b. An Liang-cheng (安良成), Wang Lin (王林), Jiang Yong-jun (江永军), et al. Effect of ZSM-5 zeolites by alkali-treatment and their catalytic performance for methanol to propylene reaction (碱改性对ZSM-5分子筛的影响及甲醇制丙烯催化性能研究) [J]. J Mol Catal (China)(分子催化), 2016, 30 (5): 444-453. |
| [6] | Liu Jun-tao(刘俊涛), Xie Zai-ku(谢在库), Xu Chun-ming(徐春明), et al. Advances in catalytic cracking of C4 olefin to propylene(C4烯烃催化裂解增产丙烯技术进展)[J]. Chem Ind Engin Pro(化工进展), 2005, 24(12): 1347–1351. DOI:10.3321/j.issn:1000-6613.2005.12.006 |
| [7] | Cao Xiang-hong(曹湘洪). Enhance the economic gain ability of petroleum refinery industry by increasing pro pylene production(增产丙烯, 提高炼化企业盈利能力)[J]. Chem Ind Engin Pro(化工进展), 2003, 22(9): 911–919. |
| [8] | Zhang Jian-jun(张建军), Zhou Yu-ming(周钰明), Yang Kang-zhen(杨抗震), et al. Research on catalytic performance of W-modified HZSM-5 catalyst for C4 olefin cracking(W-ZSM-5催化剂C4烯烃裂解制丙烯催化性能研究)[J]. J Mol Catal (China)(分子催化), 2008, 22(3): 236–241. |
| [9] | Djieugoue M A, And A M P, Kevan L. Catalytic study of methanol-to-olefins conversion in four small-pore silicoaluminophosphate molecular sieves: Influence of the structural type, nickel incorporation, nickel location, and nickel concentration[J]. J Phys Chem B, 2000, 104(27): 6452–6461. DOI:10.1021/jp000504j |
| [10] | Wragg D S, Akporiaye D, Fjellvåg H. Direct observation of catalyst behaviour under real working conditions with X-ray diffraction: Comparing SAPO-18 and SAPO-34 methanol to olefin catalysts[J]. J Catal, 2011, 279(2): 397–402. DOI:10.1016/j.jcat.2011.02.011 |
| [11] | Huang Zhi-yong(黄志永), Ke Li(柯丽), Feng Jing(冯静), et al. Study on conversion of butylenes to propylene over SAPO-34 molecular sieve(SAPO-34分子筛催化丁烯转化制丙烯的研究)[J]. J Mol Catal (China)(分子催化), 2008, 22(1): 22–26. |
| [12] | Nawaz Z, Tang X, Cui Y, et al. 1-Hexene catalytic cracking to propylene using shape selective molecular sieve SAPO-34 zeolite[J]. Arab J Fore & Engin, 2010, 35(1): 15–24. |
| [13] | Hu Y, Chen H, Hu Y, et al. Catalytic property of SAPO-18/SAPO-34 intergrown molecular sieve in 1-butene cracking[J]. Chem Lett, 2015, 44(8): 1116–1118. DOI:10.1246/cl.150289 |
| [14] | Chen J, Thomas J M, Wright P A, et al. Silicoaluminophosphate number eighteen (SAPO-18): A new microporous solid acid catalyst[J]. Catal Lett, 1994, 28(2): 241–248. |
| [15] | Lin L, Qiu C, Zhuo Z, et al. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5[J]. J Catal, 2014, 309(1): 136–145. |
| [16] | Olson D H, Kokotailo G T, Lawton S L, et al. Crystal structure and structure-related properties of ZSM-5[J]. J Phys Chem, 1981, 85(15): 2238–2243. DOI:10.1021/j150615a020 |
| [17] | Newsam J M, Treacy M M J, Koetsier W T, et al. Structural characterization of zeolite beta[J]. Proce Roy Soc A, 1988, 420(1859): 375–405. DOI:10.1098/rspa.1988.0131 |
| [18] | Chen J, Li J, Wei Y, et al. Spatial confinement effects of cage-type SAPO molecular sieves on product distribution and coke formation in methanol-to-olefin reaction[J]. Catal Commun, 2014, 46(5): 36–40. |
| [19] | Zhu X, Liu S, Song Y, et al. Catalytic cracking of 1-butene to propene and ethene on MCM-22 zeolite[J]. Appl Catal Gener, 2005, 290(1/2): 191–199. |
| [20] | Lin L F, Zhao S F, Zhang D W, et al. Acid strength controlled reaction pathways for the catalytic cracking of 1-pentene to propene over ZSM-5[J]. J Catal, 2014, 309(1): 136–145. |
| [21] | Iwase Y, Sakamoto Y, Shiga A, et al. Shape-selective catalysis determined by the volume of a zeolite cavity and the reaction mechanism for propylene production by the conversion of butene using a proton-exchanged zeolite[J]. J Phys Chem C, 2012, 116(8): 5183–5196. |
 2017, Vol. 31
2017, Vol. 31


