2. University of Chinese Academy of Sciences, No. 19 A, Yuquanlu, Beijing, 100049, China
2. 中国科学院大学, 北京 100049
Carbon materials have many specific features such as high surface area, thermal and chemical stability, and hydrophobic surface properties. In the past de-cade, there exists an increasing interest in the development of carbon supported nanocatalysts for fine chemical synthesis[1-2]. Among them, N-doped graphene has received broad attention due to its unique properties, such as tunable N or O densities, high surface area, easy-to-design structure and low energy requirements for regeneration, etc[3-5]. As is well known, cobalt-based catalysts, especially cobalt-based nanoparticles came into the focus of researchers because of their specific catalytic properties[6-10]. However, the active nano-cobalt catalysts are easily deactivated because of aggregation or leaching of the active metal during the reaction. In this respect, the development of Co@N-graphene catalysts provides a possibility to solve these problems[11-12].
N-alkyl amines are one of the key building blocks and intermediates in chemical, pharmaceutical and agrochemical industries[13-15]. Traditionally, amines and alkyl halide are typically used as the starting materials for N-alkyl amines synthesis but this procedure can be problematic due to over alkylation and the toxic nature of many alkyl halides (Scheme 1a)[16]. The use of alcohols as alkylating agents for catalytic aminations by borrowing hydrogen strategy is generally limited due to the temporary removal of hydrogen from the starting alcohol to generate aldehyde intermediate (Scheme 1b)[17-19] or less tolerant of sensitive functional groups in the starting material molecules[20]. The development of efficient approaches for C-N bond formation by selectively oxidative amination of alkanes might present an efficient method for amination reactions but it is highly challenging. Although good results were obtained in the α-amination of aldehydes and secondary sp-3-C tertiary carbons, and the cyclization reaction promoted C-H activation of aliphatic amines for strained nitrogen heterocycle synthesis[21-23], only few result was reported for the one-pot synthesis of N-benzyl aniline with aniline/nitrobenzene and toluene as the starting materials by far[24-26]. Hence, the selective functionalization of sp3-C-H bond of toluene derivatives with wide functio-nal group tolerance was highly desirable.

|
Scheme 1 Different pathways for N-alkyl amines synthesis |
Hence, in our ongoing interest in the construction of N-substituted amines[26-29], we report herein an efficient Co@N-graphene/C catalyzed oxidative sp3-C-H bond amination of toluene with nitrobenzene derivatives with the co-addition of TBHP and H2 as oxidant and reductant, which illustrates a practical straightforward route for N-alkyl amine synthesis from simple and rea-dily available starting materials (Scheme 1c).
1 Experimental 1.1 Typical procedure for Co@N-graphene preparationTypically, 0.5 mmol (124.5 mg) Co (OAc)2·4H2O and 1.5 mmol (297.3 mg) 1, 10-phenanthroline were added into 20 mL ethanol and stirred for 6 h at room temperature. Then, 615 mg carbon (XC72R) was added and the reaction mixture was stirred at room temperature for another 18 h. Then, the reaction mixture was vacuum-dried at 60 ℃ for 3 h. The obtained solid material was further dried at 60 ℃ over night, after which it was ground to fine powder. Then, the sample was placed in the feed pipe and pyrolyzed under Ar flow at 800 ℃ for 2 h. Finally, the reactor was cooled by 5 ℃/min to 300 ℃. Thereafter, from 300 ℃ to room temperature under the Ar flow. The resulting ca-talyst was ultrasonically leached hydrochloric acid (5 mol/L), washed with distilled water (1 L) and ethanol (250 mL), and dried under vacuum to obtain the Co@graphene/C catalyst (Scheme 2). The other catalysts were prepared with the same procedure. Including Co-Phen/C-NL, Co-Phen/C, Co-Phen/C-R, Cu-Phen/C, Ni-Phen/C, Fe-Phen/C, Co-Phen/FeOx-NL, Co-Phen/Al2O3-NL, Co-Phen/CeO2-NL and Co-Phen/SiO2-NL. The metal loadings are 5.6%, 1.14%, 1.12%, 0.38%, 0.56%, 0.45%, 5.5%, 4.7%, 4.6% and 4.8%(wt). The metal:C:N atomic ratios are 1:125.1:2.6, 1:285.2:5.6, 1:281.5:5.2, 1:538:7.7, 1:279.6:8.7, 1:251.4:3.7, 1:30:2.2, 1:45:1.7, 1:49:0.88 and 1:90:2.5, respectively.

|
Scheme 2 Schematic illustration of the general procedure for Co-Phen/C catalyst preparation |
XRD measurements were conducted by using a STADIP automated transmission diffractometer (STOE) equipped with an incident beam curved germanium monochromator with Cu Kα radiation. The catalyst samples were dried in air and pressed on a glass slide for analysis. The XRD patterns were scanned in the 2θ range of 10°~80°. For data interpretation, the software WinXpow (STOE) and the database of powder diffraction file (PDF) of the international centre of diffraction data (ICDD) were used. TEM characterization was carried out by using a Tecnai G2 F30 S-Twin transmission electron microscope operating at 300 kV. Single-particle EDX analysis was performed by using a Tecnai G2 F30 S-Twin Field Emission TEM in STEM mode. For TEM investigations, the catalysts were dispersed in ethanol by ultrasonication and deposited on carbon-coated copper grids. The X-ray photoelectron spectroscopy (XPS) measurements were carried out by using a VG ES-CALAB 210 instrument equipped with a dual Mg/Al anode X-ray source, a hemispherical capacitor analyzer, and a 5 keV Ar+ ion gun. All spectra were recorded by using nonmonochromatic Mg Kα (1 253.6 eV) radiation. The samples were fixed to a stainless steel sample holder by using double-sided adhesive carbon tape. The electron binding energy was referenced to the C1s peak at 284.8 eV. The peaks were fitted by Gaussian-orentzian curves after a Shirley background subtraction. For quantitative analysis, the peak area was divided by the element-specific Scofield factor and the transmission function of the analyzer. The background pressure in the chamber was less than 10-7 Pa. The loadings of all catalyst samples were determined by ICP-AES. Raman spectra were measured with 532 nm-Edge by using LabRAM HR Evolution (HORIBA Jobin Yvon S.A.S.). NMR spectra were measured by using a Bruker ARX 400 or ARX 100 spectrometer at 400 MHz (1H) and 101 MHz (13C). All spectra are reported in ppm relative to tetramethylsilane referenced to the residual solvent peaks.
1.3 Typical procedures for N-benzyl aniline synthesis and catalyst recycling0.5 mmol (61.5 mg) nitrobenzene, 5.5 mmol TBHP (tert-Butyl hydroperoxide solution, 70%. aq. soln.), 20 mg catalyst (0.76%(mol) Co) and 10 mL toluene were added in to an 80 mL autoclave. It was then exchanged with H2, and 3.0 MPa H2 was introduced. The reaction was carried out at 160 ℃ for 36 h under magnetic stirring. Subsequently, the autoclave was cooled to room temperature, and 70 mg biphenyl and 10 mL ethanol were added for quantitative analysis by GC-FID (Agilent 6890A). The catalyst was separated by centrifugation, washed by ethyl acetate and n-hexane, and used for the next run.
1.4 Purification ProcedureThe crude products were subjected to silica gel column chromatography (60, 0.053~0.038 mm supplied by Qingdao Haiyang Chemical and Special Silica Gel Co, Ltd). Eluting with about 75 mL of Petroleum Ether, followed by Petroleum Ether/EtOAc (100:1).
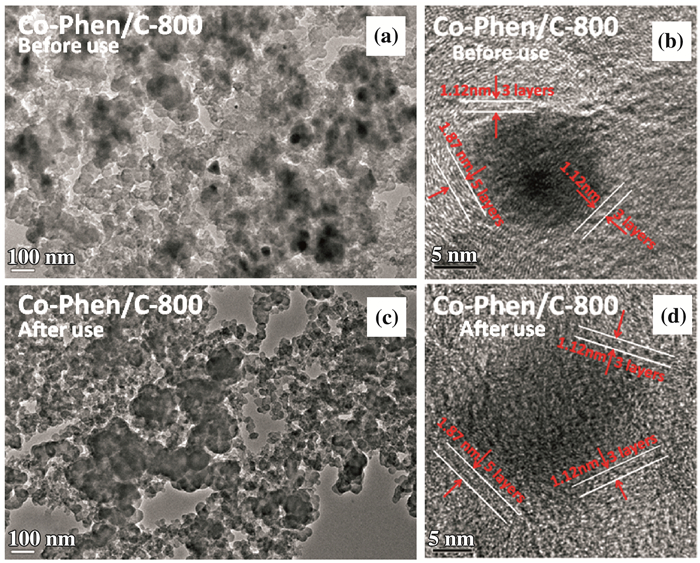
2 Results and discussionThe fresh and used Co-Phen/C catalysts were characterized by TEM and HR-TEM (Fig. 1).
|
Figure 1 TEM (a and c) and HR-TEM images (b and d) of fresh and used the Co-Phen/C |
Obviously, cobalt oxide nanoparticles with 20~50 nm particle size supported on the carbon support can be observed (Fig. 1a), and the HR-TEM mage revealed that the cobalt oxide nanoparticles were covered by graphene shell with 3~5 layers which correspond to a thickness of 1.12~1.87 nm (Fig. 1b). Interestingly, some carbon nanotubes were observed on the surface as well (Fig. 3). Most likely, the latter are left behind after removing the larger cobalt oxide particles with hydrochloric acid. It is worth noting that the core-shell structure remained intact after the catalyst was used several times, demonstrating the stability of the Co-Phen/C architecture (Fig. 1c and d).
|
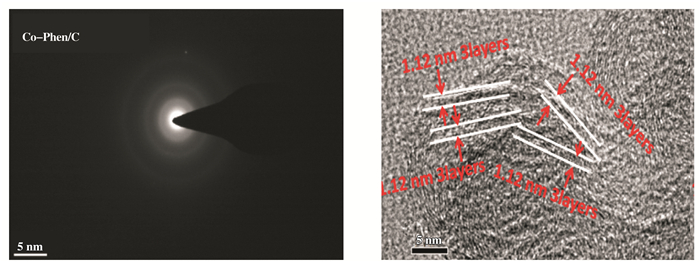
Figure 3 (SAED) patterns of Co-Phen/C (left) samples and HR-TEM image of nanotubes (right) |
The above results are consistent with the XRD characterization results (Fig. 2). The broad reflections at about 25° and 44° are from the C-support. As expected, no obvious diffraction peaks assigned to cobalt oxide nanoparticles are found from the XRD diffraction patterns of fresh and used catalysts (Fig. 2a and b), indicate that the cobalt oxide nanoparticles surrounded by a nitrogen-doped graphene shell are amorphous and stable enough. It is further confirmed by selected area electron diffraction (SAED) patterns (Fig. 3). In the local regions, the SAED patterns show only the diffraction rings of graphene, and no diffraction spots regar-ding cobalt oxide nanoparticles, which is in accordance with the results of XRD and HR-TEM.
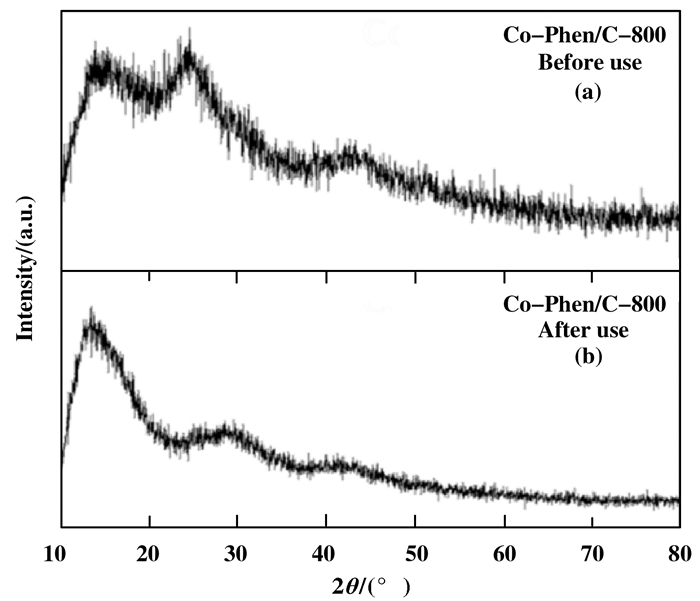
|
Figure 2 XRD patterns of fresh (a) and used (b) Co-Phen/C |
Typical XPS survey scans of Co-Phen/C catalysts are shown in Fig. 4, which reveal that the catalysts contain C, N, O and Co as the main elements. As for fresh Co-Phen/C catalyst, the peaks in the N 1s spectra at 398.9 and 400.9 eV are assigned to pyrrolic N and graphitic N (Fig. 5a)[30]. The Co 2p XPS spectra of fresh Co-Phen/C catalyst present only characteristic peaks for CoO with the typical binding energies at 780.4 and 795.3 eV (Fig. 5c)[31-32]. Additionally, O 1s spectra of fresh Co-Phen/C catalyst demonstrate the binding energies at 532.3 and 533.8 eV (Fig. 5e), which can be attributed to alcohol, ether or carbonyl groups[33-34].
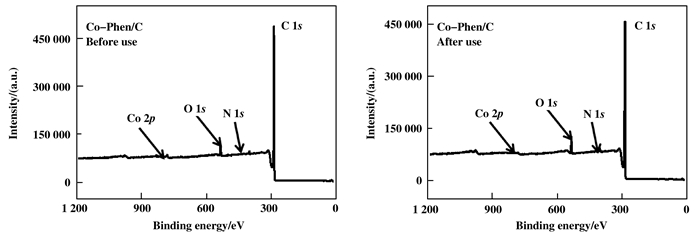
|
Figure 4 Typical XPS survey scans of Co-Phen/C samples |

|
Figure 5 N 1s XPS (a and b), Co 2p XPS (c and d) and O 1s XPS spectra (e and f) of fresh and used Co-Phen/C |
However, N-doped graphene are always a complex system because of the coexistence of various N species, and it is still under debate as to the function of diffe-rent N species. Thus, the Co-Phen/C catalyst being used for five runs was used to investigate the interaction between the N species and cobalt oxide nanoparticles as the Co-Phen/C catalyst still maintains high activity and selectivity after five cycles. By comparing the N 1s XPS spectra of Co-Phen/C catalysts before and after use (Fig. 5a and b), a noticeable shift of binding energy was observed from 399.3 to 401.1 eV, and the peak area of graphitic N turned bigger while the pyrro-lic N turned smaller. Interestingly, Co 2p spectra obviously shift toward the high-energy side, too. These results suggest the interaction between oxide cobalt nanoparticles and graphitic N (Fig. 5c and d). In addition, the Co loading dropped from 1.14% to 1.12% after five runs, showing that Co-Phen/C catalyst maintained stable and no active metal leaching occurred during the reaction. Accordingly, The O 1s spectra are not changed before and after reaction, so the electronic interaction might only occur between graphitic N and cobalt nanoparticles.
Raman spectroscopy is a powerful technique to characterize the structure and electronic properties of catalytic materials, particularly to determine the defects, and the ordered and disordered structures of carbon materials[35-36]. The Raman spectra of fresh and used Co-Phen/C were shown in Fig. 6, and two remarkable peaks at around 1 353 and 1 603 cm-1 assigned to the well-defined D and G bands were observed. The broadened D band centered at 1 353 cm-1 is caused by multiple overlapping peaks (the mode of C—N vibration at 1 285 cm-1, the mode of C=N at 1 400 cm-1 and the D mode of graphene)[37-39], whereas the G band centered at 1 603 cm-1 arises from the overlap of the G bands of graphene[40-41]. The high intensity of the D band of fresh and used Co-Phen/C clearly indicates the presence of defects in the carbon layer, which should be caused by N/O-doping in the graphene shell. Also, a broad 2D band was observed at 2 821 cm-1, revealing that typical Raman characteristics for few-layer graphene sheets with abundant defects[42-43], which should be favorable to the improvement of its electrochemical properties. Compared with fresh Co-Phen/C, the ID/IG ratio in used Co-Phen/C sample becomes smaller, which might be ascribed to the improvement of the interaction between oxide cobalt nanoparticles and graphitic N.

|
Figure 6 Raman spectra of fresh (left) and used (right) Co-Phen/C |
In order to examine the activity of the prepared catalysts for the oxidative amination of toluene, aniline and toluene were used as the model substrates first. Unfortunately, the isolated yield of N-benzyl aniline was only 31% while the conversion of aniline was 100%, which is caused by the formation of tar from aniline in the presence of the oxidant.
Thus, we have adopted the nitrobenzene instead of aniline with the addition of hydrogen. In this way, aniline can be released slowly during reaction and it might react fast with toluene in the presence of catalyst and TBHP (tert-Butyl hydroperoxide solution, 70%. aq. soln.) to avoid tar formation. So, the reductive-oxidative amination of toluene with nitrobenzene was used as the model reaction for catalyst screening and reaction conditions optimization (Table 1).
| Table 1 Results of catalyst screening and reaction conditions optimizationa |
First, 87% nitrobenzene conversion with 38% N-benzyl aniline yield was obtained if Co-Phen/C-NL, which was not being leached with hydrochloric acid, was used as catalyst (Entry 1). Noteworthy, the yield of N-benzyl aniline reached 96% if Co-Phen/C was used as the catalyst (Entry 2-4). These results suggest that the cobalt confined inside the graphene nanopores should be the catalytically active site, while cobalt oxide maintained outside of the graphene causes the side reactions. If other metals such as Cu, Ni and Fe, were used to instead of Co, only poor to moderate results were obtained (Entries 5-7). In addition, the carbon support is essential to gain the nice catalytic perfor-mance. Only 25%~52% N-benzyl aniline yields were obtained if Fe2O3, Al2O3, CeO2 and SiO2 were used as the supports (Entries 8-11). Finally, it should be mentioned that this catalyst can be easily recovered and recycled, and 95% yield was maintained when it was used in the 5th run (Entry 12). Finally, no reaction was happened in the absence of the catalyst (Entry 13).
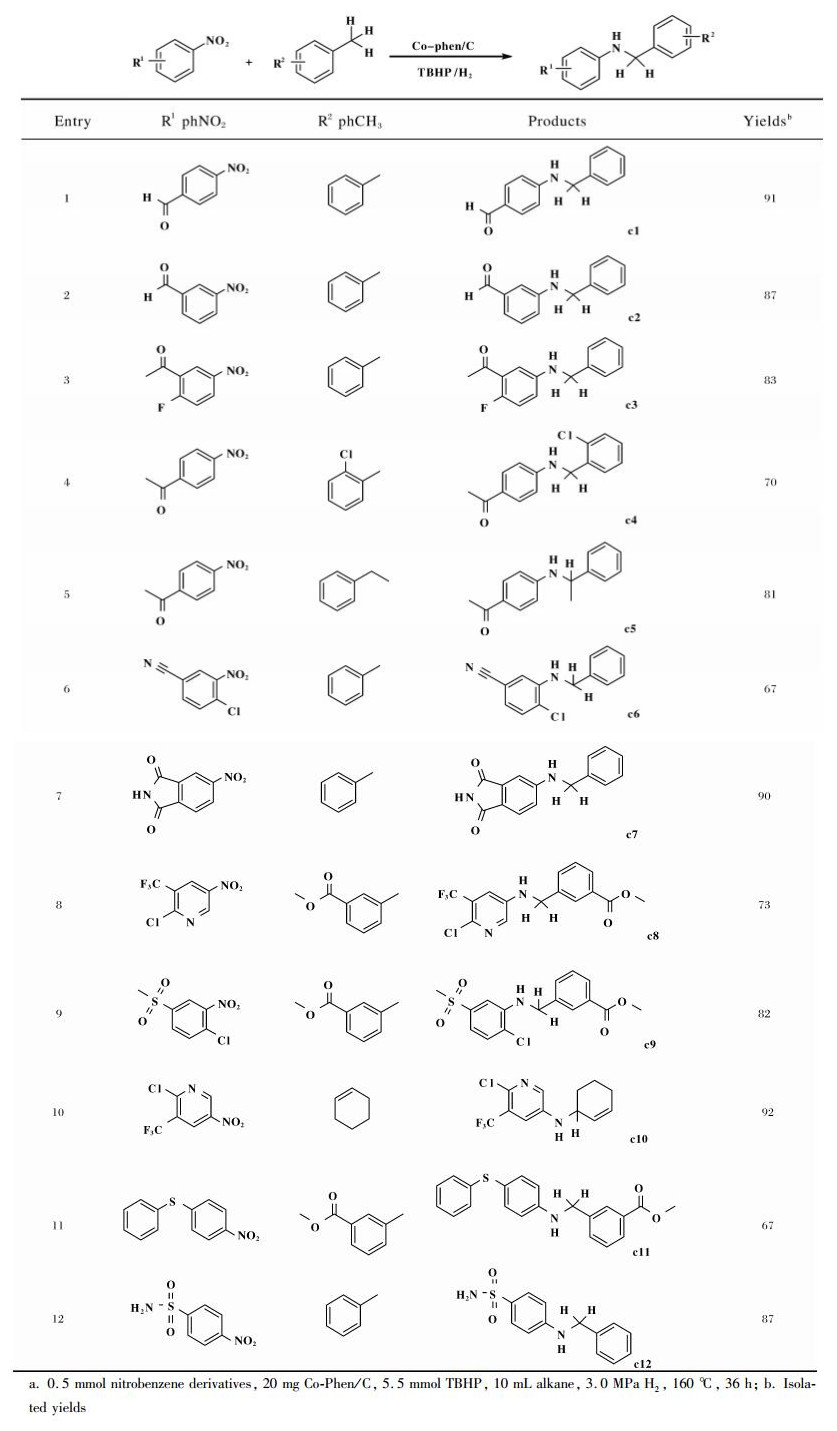
But all the while, we aimed to achieve the cataly-tically oxidative amination and overcome the lack of the approaches with wide sensitivity functional groups tole-rance (Table 2). Carbonyl is easily observable in many functional molecules; therefore, nitrobenzene derivatives with aldehyde or ketone groups were first exa-mined. To our delight, 91% and 87% isolated yields of compounds c1 and c2 were obtained, respectively (Entries 1-2). The yields of c3-c5, for which the ketone groups were at different positions of the aromatic rings of the nitrobenzene derivatives, were 70%~83% via the selective catalytic oxidative amination of toluene (Entries 3-5). Therefore, our catalyst system is sui-table for the selective catalytic oxidative amination of toluene with nitrobenzene derivatives containing aldehyde or ketone groups. Furthermore, the presence of nitrile and pyrrolidine-2, 5-dione groups do not influence the reactivity of nitrobenzene, and 67%~90% yields of c6 and c7 were obtained (Entries 6-7). The presence of a carbonyl group in the aromatic ring of to-luene is also applicable in the oxidative amination reactions. The reactions of methyl 3-methylbenzoate with 2-chloro-5-nitro-3-(trifluoromethyl) pyridine and 1-chloro-4-(methylsulfonyl)-2-nitrobenzene gave 67%~82% yields (c8-c9 entries 8-9). In addition, the presence of thioether, which is an easily oxidizable group, is also tolerated in the reaction. Compounds c10 and c11 can be synthesized with 66% and 67% yields, respectively (Entries 10-11). The amination of toluene can progress well with nitrobenzene derivatives containing sulfonamide, and up to 87% yields of c12 were obtained (Entry 12). Importantly, no reaction occurred on the sulfonamide group.
| Table 2 Reductive-oxidative amination of toluene with nitrobenzene derivativesa |
Then, the scope and generality of this catalyst in the oxidative amination of toluene derivatives were explored (Scheme 3). First, the reaction of toluene with nitrobenzene was examined, and the yield of N-benzyl aniline was 96% (c13). The steric effect can be observed when p-, o-or m-Me nitrobenzene was used as the starting material. The yields of the corresponding products were 96%, 91% and 44% (c14-c16). Only 27% yield of the desired product was obtained when 2, 6-di-Me nitrobenzene was used as the starting material (c17). Good to excellent results were also obtained if nitrobenzene derivatives with other substituting groups, such as Cl-, MeO-, Ph-, MeSO2-, Naphth-, and F3C-, were used as the starting materials. The yields of the desired products ranged from 67%~93% (c18-c28). The reaction of other alkanes can also be performed. For example, N-(1-phenylethyl) aniline can be synthesized with 71% yield via the reaction of ethyl benzene with nitrobenzene (c29). The amination of ethyl benzene was also realized with p-Ph-nitrobenzene as the nitrogen source, and 80% yield of the desired product was obtained (c30). To our delight, the selective α-amination of cyclic olefins can progress smoothly without any reduction of the double bonds, and the yields of c32-c34 reached 90%.

|
Scheme 3 Reductive-oxidative amination of nitrobenzene with toluene derivatives. The reaction conditions are the same as those in the footnote of Table 2 |
Finally, in order to gain an insight into the me-chanism of the Co-Phen/C-catalyzed oxidative amination of toluene derivatives to N-alkyl amines, isotope tracing reactions were performed with nitrobenzene and d8-toluene as the model substrates (Scheme 4). Based on the 1H NMR of the benzyl group of the final product, it can be observed that only d7-benzyl aniline was obtained at the initial stage of the reaction when it was reacted for 1 h (Scheme 4a and 5). When the reaction was prolonged to 9 h, 7.5% deuterium (determined by 1H NMR) of the-CD2-in the benzylic group was replaced by hydrogen (Scheme 4a and 5). This fin-ding should be attributed to the reversibility of the reaction (Scheme 4b). Meanwhile, It should be noted that the reactions of nitrobenzene with d8-toluene under the present reaction conditions afforded the correspon-ding d7-benzyl alcohol, d6-benzaldehyde and d14-1, 2-diphenylethane as main byproducts (Scheme 4c). These results revealed that the reaction should not progress with benzyl alcohol or aldehyde as the possible intermediate. If aldehyde was the reaction intermediate, then the reaction would take the reductive amination pathway, and at least 50% of the hydrogen atoms would be observed in the benzylic group. In addition, alcohol may not be the reaction intermediate, because only 16% of desired product was observed if benzyl alcohol was employed. Therefore, aldehyde and alcohol have not been involved into the reaction. Also, the formation of d14-1, 2-diphenylethane suggests that the reaction intermediate may be benzyl radical.
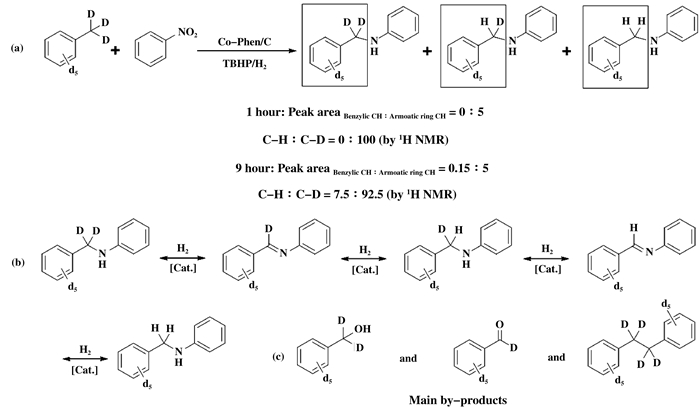
|
Scheme 4 Mechanism exploration with isotope tracing reactions |
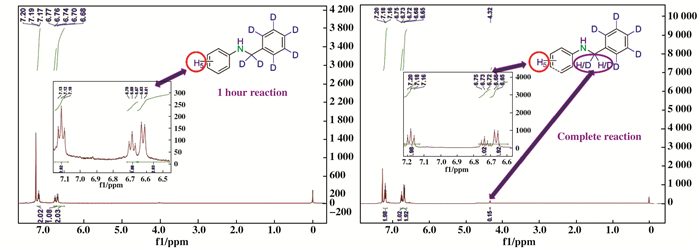
|
Scheme 5 1H NMR spectra of the product with aniline and d8-toluene as starting materials |
So, the capture of the active intermediate was examined with TEMPO as shown in Scheme 6. When 0.4 equiv., (equals to TBHP) of 2, 2, 6, 6-tetramethylpiperidyl-1-oxyl (TEMPO) was added to the reaction system, 80% yield of N-benzyl aniline can be detectable with GC-FID. While 42% yield of N-benzyl aniline was obtained if 0.8 equiv., of TEMPO was added, and no reaction can be observed if 1.2 equiv., of TEMPO was added to the reaction system. It suggests that the reaction might involve a radical process. The possible intermediate was further investigated through the capture of the free radical intermediate with TEMPO by GC/TOF-HR-MS probe, and the coupling pro-duct of benzyl radical with TEMPO was observed.
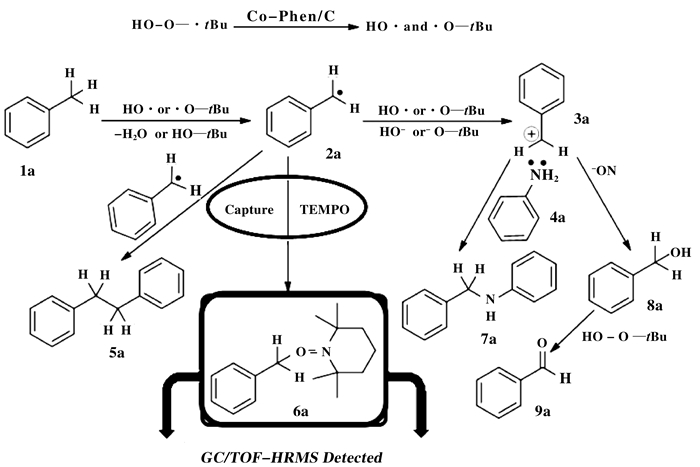
|
Scheme 6 The capture of the active intermediate |
Based on the above results, a possible reaction mechanism is proposed. Initially, the active HO·or ·O-tBu were formed via catalytic decomposition of TBHP with Co-Phen/C. The obtained radical species then reacted with toluene to generate the benzyl radical, which was further oxidized by the obtained radical species to afford benzyl cation. Finally, the desired product N-benzyl aniline was formed through a nucleophilic reaction of amine to benzyl cation.
Experimental characterization data for products:
(c1):Pale yellow oil; 1H NMR (400 MHz, CDCl3) δ 9.75 (s, 1H), 7.72 (d, J = 8.6 Hz, 2H), 7.41-7.31 (m, 5H), 6.68 (d, J = 8.6 Hz, 2H), 4.76 (s, 1H), 4.45 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 190.27, 153.07, 137.91, 132.31, 128.88, 127.71, 127.40, 126.92, 112.08, 47.59. (c2): Pale yellow oil; 1H NMR (400 MHz, CDCl3) δ 9.94 (s, 1H), 7.40-7.38 (m, 6H), 7.23 (s, 1H), 7.16 (d, J = 1.6 Hz, 1H), 6.90 (dd, J = 8.1, 1.7 Hz, 1H), 4.41 (s, 2H), 4.34 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 192.80, 148.61, 138.66, 137.57, 129.79, 128.78, 127.50, 120.20, 119.19, 111.68, 48.16. (c3): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.34 (d, J = 4.4 Hz, 4H), 7.30-7.26 (m, 1H), 7.09 (dd, J = 5.8, 3.1 Hz, 1H), 6.93 (dd, J = 10.5, 8.9 Hz, 1H), 6.73 (dt, J = 8.8, 3.5 Hz, 1H), 4.31 (s, 2H), 4.09 (s, 1H), 2.60 (d, J = 5.1 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 196.37, 196.34, 156.62, 154.19, 144.43, 138.74, 128.73, 127.53, 125.86, 125.72, 118.94, 118.86, 117.37, 117.12, 112.73, 48.71, 31.49, 31.41. (c4): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.84 (d, J = 8.3 Hz, 2H), 7.38 (dd, J = 12.6, 7.5 Hz, 2H), 7.28-7.22 (m, 2H), 6.61 (d, J = 8.4 Hz, 2H), 4.95 (s, 1H), 4.53 (s, 2H), 2.51 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 196.34, 151.66, 135.54, 133.33, 130.80, 129.76, 128.79, 127.07, 111.75, 45.32, 26.01. (c5): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 8.8 Hz, 2H), 7.36 (d, J = 4.3 Hz, 5H), 6.51 (d, J = 8.7 Hz, 2H), 4.73 (s, 1H), 4.60 (q, J = 6.7 Hz, 1H), 2.47 (s, 3H), 1.58 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 196.37, 151.20, 144.11, 130.82, 130.66, 128.84, 127.24, 125.73, 112.17, 53.09, 25.97, 24.67. (c6): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.35 (dd, J = 7.6, 5.4 Hz, 6H), 6.95-6.89 (m, 1H), 6.81 (d, J = 1.7 Hz, 1H), 4.95 (s, 1H), 4.40 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 144.29, 137.30, 129.81, 128.99, 127.86, 127.22, 120.78, 113.80, 47.64. (c7): Yellow solid; m.p. = 207~210 ℃; 1H NMR (400 MHz, DMSO) δ 10.75 (s, 1H), 7.56 (t, J = 5.9 Hz, 1H), 7.47 (d, J = 8.3 Hz, 1H), 7.40-7.30 (m, 4H), 7.26 (td, J = 5.8, 2.7 Hz, 1H), 6.86 (dd, J = 11.3, 3.0 Hz, 2H), 4.42 (d, J = 5.9 Hz, 2H).13C NMR (101 MHz, DMSO) δ 170.07, 169.73, 154.41, 139.26, 135.80, 128.96, 127.64, 127.47, 124.95, 118.72, 116.23, 105.67, 46.50. (c8): Pale yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.99-7.89 (m, 2H), 7.61-7.42 (m, 3H), 7.21 (d, J = 2.8 Hz, 1H), 4.78 (s, 2H), 4.44 (s, 1H), 3.94 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 141.24, 131.39, 129.21, 129.14, 128.81, 128.65, 128.42, 127.96, 52.28, 52.15, 47.57. (c9): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 6.5 Hz, 2H), 7.44-7.36 (m, 3H), 7.15 (m, 2H), 5.13 (s, 1H), 4.48 (s, 2H), 3.89 (d, J = 1.7 Hz, 3H), 2.95 (d, J = 1.5 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 144.26, 140.00, 138.00, 131.93, 131.38, 129.95, 128.99, 128.52, 127.88, 124.44, 115.83, 109.17, 52.22, 52.10, 47.28, 44.33. (c10): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.90 (d, J = 2.7 Hz, 1H), 7.17 (d, J = 2.9 Hz, 1H), 5.94 (d, J = 10.0 Hz, 1H), 5.76-5.60 (m, 1H), 3.98 (s, 2H), 2.06 (s, 2H), 1.96-1.86 (m, 1H), 1.73-1.61 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 142.08, 136.85, 131.77, 126.56, 119.29, 47.87, 28.31, 24.91, 19.32. (c11): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.93 (d, J = 8.5 Hz, 4H), 7.56-7.25 (m, 9H), 3.91 (d, J = 8.0 Hz, 3H), 2.44 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 171.40, 138.28, 134.44, 130.68, 128.37, 127.33, 52.11, 21.25. (c12): Yellow solid; m.p. = 169~172oC; 1H NMR (400 MHz, DMSO) δ 6.52 (d, J = 8.8 Hz, 2H), 6.39-6.31 (m, 5H), 6.03 (t, J = 5.5 Hz, 1H), 5.93 (s, 2H), 5.67 (d, J = 8.8 Hz, 2H), 3.37 (d, J = 5.4 Hz, 2H). 13C NMR (101 MHz, DMSO) δ 151.92, 140.06, 130.97, 129.04, 127.98, 127.77, 127.46, 111.79, 46.55. (c13): White solid; m.p. = 39 oC; 1H NMR (400 MHz, CDCl3) δ 7.35 (td, J = 8.0, 1.5 Hz, 4H), 7.30-7.24 (m, 1H), 7.21-7.11 (m, 2H), 6.72 (t, J = 7.3 Hz, 1H), 6.67-6.58 (m, 2H), 4.33 (s, 2H), 4.12 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 148.09, 139.40, 129.28, 128.65, 127.55, 127.26, 117.66, 112.94, 48.41. (c14): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.39-7.27 (m, 4H), 7.25 (t, J = 6.9 Hz, 1H), 6.97 (d, J = 8.3 Hz, 2H), 6.55 (d, J = 8.4 Hz, 2H), 4.28 (s, 2H), 3.88 (s, 1H), 2.22 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 145.94, 139.71, 129.80, 128.65, 127.57, 127.21, 126.84, 113.12, 48.73, 20.45. (c15): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.38-7.25 (m, 5H), 7.05 (m, J = 10.0, 7.6, 2.2 Hz, 1H), 6.54 (s, 1H), 6.43 (d, J = 13.1 Hz, 2H), 4.29 (s, 2H), 3.94 (s, 1H), 2.26 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 148.31, 139.66, 139.10, 129.23, 128.69, 127.61, 127.27, 118.64, 113.76, 110.09, 48.45, 21.71. (c16): White solid; m.p. = 55 ~ 56 ℃; 1H NMR (400 MHz, CDCl3) δ 7.43-7.32 (m, 5H), 7.16-7.09 (m, 2H), 6.73 (t, J = 7.4 Hz, 1H), 6.67 (d, J = 8.0 Hz, 1H), 4.42 (s, 2H), 4.12 (s, 1H), 2.22 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 145.94, 139.42, 130.12, 128.68, 127.61, 127.30, 127.19, 122.08, 117.37, 110.20, 48.44, 17.56. (c17): Yellow solid; m.p. = 78 ~ 84oC; 13C NMR (101 MHz, CDCl3) δ 138.80, 134.36, 128.75, 127.91, 127.51, 126.59, 125.83, 124.91, 123.57, 120.02, 118.17, 48.92. 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 1.6 Hz, 2H), 7.36 (s, 2H), 7.06 (s, 1H), 7.00 (s, 2H), 6.88 (d, J = 7.3 Hz, 1H), 4.15 (s, 2H), 3.48 (s, 1H), 2.28 (s, 6H). (c18): Yellow solid; m.p. = 45oC; 1H NMR (400 MHz, CDCl3) δ 7.33 (d, J = 4.4 Hz, 4H), 7.26 (dd, J = 6.2, 3.1 Hz, 1H), 7.09 (d, J = 8.9 Hz, 2H), 6.52 (d, J = 8.8 Hz, 2H), 4.28 (s, 2H), 4.08 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 146.66, 138.96, 129.11, 128.74, 127.46, 127.41, 122.19, 114.00, 48.41. (c19): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.35-7.24 (m, 5H), 7.03 (t, J = 8.0 Hz, 1H), 6.73-6.60 (m, 1H), 6.58 (t, J = 2.0 Hz, 1H), 6.45 (dd, J = 8.2, 2.0 Hz, 1H), 4.26 (s, 2H), 4.06 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 149.29, 138.82, 135.09, 130.27, 128.79, 127.52, 127.49, 117.47, 112.59, 111.20, 48.15. (c20): Yellow solid; m.p. = 50 ℃; 1H NMR (400 MHz, CDCl3) δ 7.34 (q, J = 7.1 Hz, 4H), 7.26 (dd, J = 12.8, 6.4 Hz, 1H), 6.83-6.72 (m, 2H), 6.60 (d, J = 8.7 Hz, 2H), 4.27 (s, 2H), 3.73 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 152.30, 142.44, 139.70, 128.62, 127.59, 127.20, 114.97, 114.21, 55.84, 49.32. (c21): Pale yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.39-7.32 (m, 3H), 7.27 (d, J = 6.8 Hz, 1H), 7.23-7.13 (m, 1H), 6.87-6.73 (m, 2H), 6.67 (td, J = 7.8, 1.4 Hz, 1H), 6.59 (d, J = 7.8 Hz, 1H), 4.60 (s, 1H), 4.34 (s, 2H), 3.83 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 146.86, 139.62, 138.17, 128.60, 127.55, 127.14, 121.33, 116.69, 110.16, 109.48, 55.45, 48.10. (c22): Yellow solid; m.p. = 109 ~ 110 ℃; 1H NMR (400 MHz, CDCl3) δ 7.44 (dd, J = 4.9, 1.7 Hz, 1H), 7.37 (d, J = 4.5 Hz, 4H), 7.32-7.30 (m, 1H), 6.81 (d, J = 8.8 Hz, 1H), 6.74 (d, J = 2.8 Hz, 1H), 6.52 (dd, J = 8.8, 2.8 Hz, 1H), 4.28 (s, 2H), 4.06 (s, 1H), 3.83 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 147.62, 142.85, 138.97, 128.69, 127.56, 127.39, 115.22, 114.33, 112.14, 57.07, 49.03. (c23): Pale yellow solid; m.p. = 47 ~ 50 ℃; 1H NMR (400 MHz, CDCl3) δ 7.40-7.29 (m, 6H), 6.69 (s, 1H), 4.72 (d, J = 10.7 Hz, 1H), 4.35 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 143.33, 137.60, 131.73, 129.87, 128.94, 127.78, 127.33, 119.46, 117.78, 112.26, 47.89. (c24): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.44 (d, J = 8.1 Hz, 1H), 7.38 (d, J = 3.8 Hz, 4H), 7.32 (dd, J = 8.9, 4.4 Hz, 1H), 7.21-7.14 (m, 2H), 5.02 (s, 1H), 4.45 (s, 2H), 2.96 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 144.44, 140.07, 137.42, 129.86, 128.93, 127.83, 127.53, 124.31, 115.59, 109.29, 47.73, 44.41. (c25): Pale yellow solid; m.p. = 94~96 ℃; 1H NMR (400 MHz, CDCl3) δ 7.64-7.60 (m, 2H), 7.54-7.44 (m, 8H), 7.36 (dd, J = 12.3, 7.2 Hz, 2H), 6.78 (d, J = 8.5 Hz, 2H), 4.44 (s, 2H), 4.20 (s, 1H). 13C NMR (101 MHz, CDCl3) δ 147.59, 141.30, 139.36, 130.59, 128.75, 128.72, 128.02, 127.57, 127.37, 126.37, 126.15, 113.26, 48.42. (c26): White solid; m.p. = 88-90 ℃; 1H NMR (400 MHz, CDCl3) δ 7.47-7.40 (m, 4H), 7.29 (dd, J = 4.2, 2.3 Hz, 5H), 7.23 (d, J = 2.5 Hz, 1H), 7.17 (d, J = 2.4 Hz, 1H), 7.12-7.08 (m, 1H), 6.81-6.71 (m, 1H), 6.65 (d, J = 5.5 Hz, 1H), 4.46 (s, 1H), 4.31 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 144.97, 139.56, 130.30, 129.46, 129.00, 128.79, 128.65, 127.32, 127.11, 117.27, 110.85, 48.22. (c27): Yellow solid; m.p. = 65 ℃; 1H NMR (400 MHz, CDCl3) δ 7.81 (dd, J = 12.0, 8.0 Hz, 2H), 7.41-7.24 (m, 9H), 6.65 (d, J = 7.1 Hz, 1H), 4.65 (s, 1H), 4.48 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 138.80, 134.36, 128.75, 127.91, 127.51, 126.59, 125.83, 124.91, 123.57, 120.02, 118.17, 48.92. (c28): Brown oil; 1H NMR (400 MHz, CDCl3) δ 7.41 (d, J = 4.2 Hz, 4H), 7.31 (d, J = 8.4 Hz, 1H), 7.25 (d, J = 7.4 Hz, 1H), 7.02 (d, J = 7.6 Hz, 1H), 6.93 (s, 1H), 6.82 (d, J = 8.0 Hz, 1H), 4.74 (d, J = 9.2 Hz, 1H), 4.39 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 147.81, 138.28, 129.72, 128.81, 128.49, 128.37, 127.67, 116.17, 114.39, 109.55, 48.45. (c29): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.33-7.27 (m, 4H), 7.16 (dd, J = 6.5, 1.9 Hz, 1H), 7.11-7.05 (m, 2H), 6.64 (d, J = 8.0 Hz, 1H), 6.53 (d, J = 7.8 Hz, 2H), 4.48 (q, J = 6.7 Hz, 1H), 3.87 (s, 1H), 1.52 (d, J = 6.7 Hz, 3H). 13C NMR (101 MHz, CDCl3) δ 146.86, 144.93, 129.13, 128.67, 127.59, 126.98, 125.98, 117.77, 113.05, 53.88, 24.83. (c30): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.60-7.56 (m, 2H), 7.55-7.49 (m, 2H), 7.45 (dd, J = 6.2, 4.0 Hz, 6H), 7.33 (dd, J = 3.6, 2.0 Hz, 2H), 6.67 (dd, J = 6.8, 1.8 Hz, 2H), 4.61 (d, J = 6.4 Hz, 1H), 4.20 (s, 1H), 1.68-1.48 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 146.74, 145.16, 141.32, 128.78, 128.76, 128.69, 128.03, 127.87, 126.38, 126.32, 125.94, 113.69, 113.22, 53.62, 48.39, 25.04. (c31): Pale yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.99 (d, J = 7.7 Hz, 1H), 7.69 (d, J = 8.5 Hz, 1H), 7.63 (d, J = 2.2 Hz, 1H), 7.55 (s, 2H), 7.43 (dd, J = 15.4, 7.7 Hz, 6H), 7.28 (s, 1H), 6.72 (d, J = 8.6 Hz, 2H), 4.45 (s, 2H), 4.00 (s, 1H), 3.95 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 147.27, 141.17, 139.91, 131.93, 128.80, 128.67, 128.56, 128.53, 127.98, 126.32, 113.24, 52.18, 48.01. (c32): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 7.9 Hz, 2H), 7.44 (d, J = 8.5 Hz, 2H), 7.38 (t, J = 7.6 Hz, 2H), 7.27-7.22 (m, 1H), 6.70 (d, J = 8.5 Hz, 2H), 6.08-5.51 (m, 2H), 4.71-4.42 (m, 1H), 3.72 (s, 1H), 2.36 (dd, J = 9.5, 5.4 Hz, 2H), 1.79-1.58 (m, 2H). 13C NMR (101 MHz, CDCl3) δ 147.10, 141.33, 134.37, 131.81, 130.20, 128.66, 127.98, 126.30, 126.04, 59.56, 31.30, 31.23. (c33): Yellow oil; 1H NMR (400 MHz, CDCl3) δ 7.16 (dd, J = 8.3, 7.5 Hz, 2H), 6.83-6.46 (m, 3H), 5.86-5.81 (m, 1H), 5.74 (d, J = 2.6 Hz, 1H), 3.99 (s, 1H), 3.69 (s, 1H), 2.02 (dd, J = 5.4, 2.2 Hz, 2H), 1.89 (dd, J = 7.4, 4.0 Hz, 1H), 1.72-1.62 (m, 3H). 13C NMR (101 MHz, CDCl3) δ 147.17, 130.13, 129.32, 128.60, 117.21, 113.31, 47.96, 28.93, 25.18, 19.69. (c34): Yellow solid; m.p. = 189~193 ℃; 1H NMR (400 MHz, CDCl3) δ 10.70 (s, 1H), 7.44 (d, J = 8.3 Hz, 1H), 6.87 (m, J = 10.3, 4.6 Hz, 3H), 6.41-5.49 (m, 2H), 4.55 (dd, J = 4.4, 2.1 Hz, 1H), 2.47-2.38 (m, 1H), 2.35-2.19 (m, 2H), 1.57-1.52 (m, 1H). 13C NMR (101 MHz, CDCl3) δ 170.11, 169.75, 153.91, 135.88, 134.48, 131.50, 125.00, 118.19, 58.79, 32.71, 31.35, 30.79, 24.11.
3 ConclusionsIn summary, we demonstrated that carbon supported core-shell nanostructured materials, i.e., Co@N-graphene/C, can be an active catalyst for the reductive-oxidative amination of toluene and nitrobenzene with the co-addition of TBHP and H2. The optimized catalyst system allows the synthesis of N-alkyl amines with excellent functional groups tolerance including pharmaceutically relevant groups such as carbonyl, olefin, nitrile, thioether, amide, sulfonamide and others. Furthermore, mechanistic study proves that the reaction progresses via benzyl radical. This methodology may promote the development of active heterogeneous catalysts for C—H functionalization.
| [1] |
a. Julkapli N M, Bagheri S. Graphene supported heterogeneous catalysts: An overview[J]. Int J Hydro Ener, 2013, 40(2): 948-979. b. Ma Lin (马琳), Kang Xiao-xue (康晓雪)), Hu Shao-zheng (胡绍争), et al. Preparation of Fe, P Co-dopedGraphitic carbon nitride with enhanced visible-light photocatalytic activity (Fe-P共掺杂石墨相氮化碳催化剂可见光下催化性能研究)[J]. J Mol Catal(China)(分子催化), 2015, 29(4): 359-368. c. Ni Jun (倪军), Luo Xiao-fang (罗小芳), Zhan Yong (詹勇), et al. Application and progress of the novel activated carbon in the field of catalysis (新型碳材料在催化领域中的应用及进展)[J]. J Mol Catal(China)(分子催化), 2016, 30(3): 282-296. d. Yang Jian (杨建), Niu Li-hong (牛丽红), Zhang Zhi-jun (张治军). Study electrocatalytic performance for oxygen reduction reaction of dopamine derived transition metal-nitrogen codoped carbon nanotube (多巴胺为前驱体过渡金属与N共同掺杂的碳纳米管催化剂ORR性能研究)[J]. J Mol Catal(China)(分子催化), 2016, 30(5): 409-419. |
| [2] |
a. Morales-Torres S, Maldonado-Hódar F J, Pérez-Cadenas A F, et al. Coupling noble metals and carbon supports in the development of combustion catalysts for the abatement of BTX compounds in air streams[J]. Catal, 2015, 5(2): 774-799. b. Liu Pan (刘攀), Lu Ji-chang (陆继长), Chen Ding-kai (陈定凯), et al. Research of hydrogen production by thermocatalytic decomposition of methane on carbonaceous and metal catalysts (碳质与金属催化剂热催化裂解甲烷产氢研究进展)[J]. J Mol Catal(China)(分子催化), 2016, 30(5): 480-495. |
| [3] | Lu Y, Chen Y, Zhang M, et al. Nitrogen-doped graphene materials for supercapacitor applications[J]. J Nanosci Nanotechnol, 2014, 14(2): 1134–1144. DOI:10.1166/jnn.2014.9102 |
| [4] | Xiong H, Coville N J, Moyo M, et al. Fischer-tropsch synthesis:Iron-based catalysts supported on nitrogen-doped carbon nanotubes synthesized by post-doping[J]. Appl Catal, A, 2014, 482: 377–386. DOI:10.1016/j.apcata.2014.06.019 |
| [5] | Nongwe I, Coville N J, Meijboom R, et al. Pt supported nitrogen doped hollow carbon spheres for the catalysed reduction of cinnamaldehyde[J]. Appl Catal, A, 2016, 517: 30–38. DOI:10.1016/j.apcata.2016.02.025 |
| [6] | Yan J, Kung H H, Sachtler W M H, et al. Co/Al2O3 lean NOx reduction catalyst[J]. J Catal, 1997, 172(1): 178–186. DOI:10.1006/jcat.1997.1869 |
| [7] | Zhao Hong(赵虹), Ma Li-yong(马利勇). Synthesis of cobalt molecular sieve catalyst and influence of the support on the selective oxidation of cyclohexane(钴分子筛催化剂的制备及其分子筛载体对环己烷氧化选择性的影响)[J]. J Mol Catal(分子催化), 2006, 20(4): 316–321. |
| [8] | Surendranath Y, Dinca M, Nocera D G. Electrolyte-dependent electrosynthesis and activity of cobalt-based water oxidation catalysts[J]. J Am Chem Soc, 2009, 131(7): 2615–2620. DOI:10.1021/ja807769r |
| [9] | Zhou Yu-lu(周玉路), Lin Sha-sha(林莎莎). Oxidation of ethylbenzene catalyzed by NHPI combined with cobalt sulfonated phthalocyanine(NHPI结合磺化酞菁钴催化的乙苯氧化反应)[J]. J Mol Catal(分子催化), 2006, 25(6): 508–513. |
| [10] | Feng Ze(冯泽), Liu Ping-le(刘平乐). The catalytic performance of cobalt tetra (4-nitrophonyl) metalloporphyrin supported on zinc oxide in cyclohexane oxidation(氧化锌负载四 (4-硝基苯基) 钴卟啉催化氧化环己烷性能)[J]. J Mol Catal(分子催化), 2016, 30(1): 20–28. |
| [11] | Banerjee D, Beller M, Junge K, et al. Convenient and mild epoxidation of alkenes using heterogeneous cobalt oxide catalysts[J]. Angew Chem Int Ed, 2014, 53(17): 4359–4363. DOI:10.1002/anie.201310420 |
| [12] | Jagadeesh R V, Beller M, Arockiam P B, et al. Highly selective transfer hydrogenation of functionalised nitroarenes using cobalt-based nanocatalysts[J]. Green Chem, 2015, 17(2): 898–902. DOI:10.1039/C4GC00731J |
| [13] | Lawrence S A. Amines:Synthesis, properties and applications[M]. Cambridge University Press, 2004. |
| [14] | Maxwell G. Synthetic nitrogen products:A practical guide to the products and processes[M]. Springer Science & Business Media, 2004. |
| [15] | Rappoport Z. The chemistry of anilines[M]. John Wiley & Sons, 2007. |
| [16] | Salvatore R N, Yoon C H, Jung K W. Synthesis of secondary amines[J]. Tetrahedron, 2001, 57(37): 7785–7811. DOI:10.1016/S0040-4020(01)00722-0 |
| [17] | Watson A J, Williams J M. The give and take of alcohol activation[J]. Science, 2010, 329(5992): 635–636. DOI:10.1126/science.1191843 |
| [18] | Bähn S, Beller M, Neubert L, et al. The catalytic amination of alcohols[J]. Chem Cat Chem, 2011, 3(12): 1853–1864. |
| [19] | Pang S, Deng Y, Shi F. Synthesis of unsymmetric tertiary amines via alcohol amination[J]. Chem Commun, 2015, 51(46): 9471–9474. DOI:10.1039/C5CC02205C |
| [20] | Cui X, Shi F, Deng Y, et al. Development of a general non-noble metal catalyst for the benign amination of alcohols with amines and ammonia[J]. Chem Eur J, 2013, 19(11): 3665–3675. DOI:10.1002/chem.v19.11 |
| [21] | Pan J, Su M, Buchwald S L. Palladium (0)-catalyzed intermolecular amination of unactivated C Sp3-H bonds[J]. Angew Chem Int Ed, 2011, 50(37): 8647–8651. DOI:10.1002/anie.201102880 |
| [22] | Tian J S, Loh T P, Wong J R, et al. α-amination of aldehydes catalyzed by in situ generated hypoiodite[J]. Angew Chem Int Ed, 2012, 51(36): 9105–9109. DOI:10.1002/anie.201204215 |
| [23] | McNally A, Haffemayer B, Collins B S L, et al. Palladium-catalysed CH activation of aliphatic amines to give strained nitrogen heterocycles[J]. Nature, 2014, 510(7503): 129–133. DOI:10.1038/nature13389 |
| [24] | Gephart R T, Warren T H, Aguila M J B, et al. Catalytic C-H amination with aromatic amines[J]. Angew Chem Int Ed, 2012, 51(26): 6488–6493. DOI:10.1002/anie.201201921 |
| [25] | Zhang X, Wang L, Li P, et al. n-Bu4NI/TBHP-catalyzed direct amination of allylic and benzylic C (sp3)-H with anilines under metal-free conditions[J]. Chem Commun, 2014, 50(59): 8006–8009. DOI:10.1039/c4cc01189a |
| [26] | Pang S, Shi F. Oxidative amination of benzylic alkanes with nitrobenzene derivatives as nitrogen sources[J]. Tetra Lett, 2016, 57(52): 5872–5876. DOI:10.1016/j.tetlet.2016.11.057 |
| [27] | Shi F, Beller M, Cui X, et al. Copper-catalyzed alkylation of sulfonamides with alcohols[J]. Angew Chem Int Ed, 2009, 48(32): 5912–5915. DOI:10.1002/anie.v48:32 |
| [28] | Cui X, Zhang Y, Shi F, et al. Ruthenium-catalyzed nitro and nitrile compounds coupling with alcohols:Alternative route for N-substituted amine synthesis[J]. Chem Eur J, 2011, 17(9): 2587–2591. DOI:10.1002/chem.201003095 |
| [29] | Yang H, Cui X, Shi F, et al. Carbon-catalysed reductive hydrogen atom transfer reactions[J]. Nat Commun, 2015, 6. |
| [30] | Cui X, Li Y, Beller M, et al. Synthesis and characterization of iron-nitrogen-doped graphene/core-shell catalysts:Efficient oxidative dehydrogenation of N-heterocycles[J]. J Am Chem Soc, 2015, 137(33): 10652–10658. |
| [31] | Lee K, Zhang L, Zhang J. Ternary non-noble metal chalcogenide (W-Co-Se) as electrocatalyst for oxygen reduction reaction[J]. Electrochem Commun, 2007, 9(7): 1704–1708. DOI:10.1016/j.elecom.2007.03.025 |
| [32] | Wen Y, Zhang C, Teraoka Y, et al. Catalytic oxidation of nitrogen monoxide over La1-xCexCoO3 Perovskites[J]. Catal Today, 2007, 126(3): 400–405. |
| [33] | Amor S B, Baud G, Nardin M, et al. XPS characterisation of plasma-treated and alumina-coated pmma[J]. Appl Surf Sci, 2000, 153(2): 172–183. |
| [34] | Stevens J S, Schroeder S L. Quantitative analysis of saccharides by X-Ray photoelectron spectroscopy[J]. Surf Interf Anal, 2009, 41(6): 453–462. DOI:10.1002/sia.v41:6 |
| [35] | Ferrari A C, Robertson J. Interpretation of raman spectra of disordered and amorphous carbon[J]. Phys Rev B, 2000, 61(20): 14095. DOI:10.1103/PhysRevB.61.14095 |
| [36] | Sadezky A, Muckenhuber H, Pöschl U, et al. Raman microspectroscopy of soot and related carbonaceous materials:Spectral analysis and structural information[J]. Carbon, 2005, 43(8): 1731–1742. DOI:10.1016/j.carbon.2005.02.018 |
| [37] | Beyssac O, Goffé B, Rouzaud J N, et al. On the characterization of disordered and heterogeneous carbonaceous materials by raman spectroscopy[J]. Spectrochim Acta, Part A, 2003, 59(10): 2267–2276. DOI:10.1016/S1386-1425(03)00070-2 |
| [38] | Shan C, Yang H, Niu L, et al. Water-soluble graphene covalently functionalized by biocompatible Poly-L-Lysine[J]. Langmuir, 2009, 25(20): 12030–12033. DOI:10.1021/la903265p |
| [39] | Jeon I Y, Choi H J, Shin D, et al. Direct nitrogen fixation at the edges of graphene nanoplatelets as efficient electrocatalysts for energy conversion[J]. Sci Rep, 2013, 3: 2260. DOI:10.1038/srep02260 |
| [40] | Guo B, Liu Q, Gong J R, et al. Controllable N-doping of graphene[J]. Nano Lett, 2010, 10(12): 4975–4980. DOI:10.1021/nl103079j |
| [41] | Georgakilas V, Otyepka M, Kim K S, et al. Functionalization of graphene:Covalent and non-covalent approaches, derivatives and applications[J]. Chem Rev, 2012, 112(11): 6156–6214. DOI:10.1021/cr3000412 |
| [42] | Sutter P W, Flege J L, Sutter E A, et al. Epitaxial graphene on ruthenium[J]. Nat Mater, 2008, 7(5): 406–411. DOI:10.1038/nmat2166 |
| [43] | Frank O, Mohr M, Papagelis K, et al. Raman 2d-band splitting in graphene:Theory and experiment[J]. ACS Nano, 2011, 5(3): 2231–2239. DOI:10.1021/nn103493g |
 2017, Vol. 31
2017, Vol. 31