2. University of Chinese Academy of Sciences, Beij ing 100049, China
2. 中国科学院大学, 北京 100049
Diesters of maleic and fumaric acids are commodity chemicals with multiple applications[1-5]: as monomers for polymer synthesis, production of chemical intermediates, pharmaceuticals, food additives and biocide. These diesters are commercially produced by esterification of maleic anhydride which is employing petroleum-derived chemicals (Scheme 1) such as n-butane and benzene as a raw material[6]. However, the energy crisis motivated to develop a potential route for the direct synthesis of dicarboxylic acid ester to replace the route of petroleum. Carbon monoxide is an attractive carbon resource for exploitation in chemical transformations[7-9]. Especially, its use as C1 building block in carbon-carbon bond-forming reactions would open new routes for the direct synthesis of carboxylic acids and their derivatives.

|
Scheme 1 Synthesis of diesters of maleic and fumaric acids |
Dicarbonylation of acetylene was a synthetic route, which can directly employ the CO to synthesize the diesters of maleic and fumaric acids. In 1964, Tsuji and co-workers[10] showed the palladium-mediated transformation of acetylene into muconyl, fumaryl, and maleic acid chloride. While the presence of stoichiometric amounts of palladium limited its application. Then, G. P. Chiusoli[11] found that oxygen as an oxidizing agent could reoxidize Pd(0) to regenerate the catalytic cycle. Howard Alper et al[12] further improved the catalytic system using PdCl2/CuCl2/HCl and oxygen, the catalytic activity was increased. Moreover, the results indicated that CuCl2 and oxygen played the essential roles. Xu et al[13-14] also discovered the ana-logous results using the catalytic system PdCl2/FeCl3/H2SO4 and oxygen, the diester total yield was 74% (based on acetylene) at normal pressure and 60 ℃. However, concentrated HCl or H2SO4 and the heavy metallic salts (FeCl3 and CuCl2) were employed to make the reaction run smoothly. Therefore, it is desi-rable to construct an environmentally friendly and high efficient catalytic system.
Herein, we employed the PdCl2/KI/air catalytic system for dicarbonylation of acetylene, which avoided to use the large amounts of acid and heavy metallic salts. This catalytic system presented much higher ca-talytic activity than others. Moreover, the orthogonal experiments demonstrated that the partial pressure of air and the amounts of KI are the crucial roles in the reaction process.
1 Experimental 1.1 Materials and instrumentsAll chemicals were analytical grade and used directly without any further purification. The purity of acetylene and carbon monoxide was 99.99% and 99.9%, respectively. Gas chromatography analyses were performed on a SP-6800A Series GC instrument with a FID detector and a FFAP capillary chromatographic column (50 m × 0.32 mm × 0.50 μm) using the internal standard method. 1H and 13C NMR data were acquired using an INOVA-400MHz spectrometer in CDCl3 as solvent and the chemical shifts are expressed in δ units with Me4Si as internal standard. GC-MS spectra were recorded on an Agilent 7890B-5977A gas chromatography-mass spectrometry instrument.
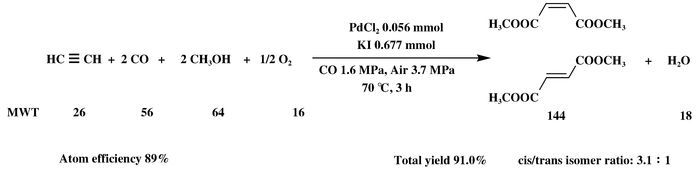
1.2 General ProcedureAll reactions were carried out in a 75 mL stainless steel autoclave. In a typical experiment (Scheme 2), PdCl2 (0.056 mmol) and KI (0.677 mmol) were added into 30 mL methanol. Then, Acetylene (0.42 g, 16.13 mmol) was slowly dissolved into the above mixture, and air (3.7 MPa) and carbon monoxide (up to 5.3 MPa of total pressure) were introduced and the mixture was stirred at 70 ℃ for 3 h. After the reaction system was cooled to room temperature, the products were filtered and quantitatively analysed by gas chromatography using the internal standard method.
|
Scheme 2 The typical catalytic dicarbonylaton of acetylene to diesters |
1H NMR (400 MHz, CDCl3) δ 6.22 (s, 2H), 3.76 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 165.7, 129.7, 52.2.
MS (EI): m/z (%): 144(1) [M]+, 113(100), 85(13), 59(17).
1.3.2 Dimethyl fumarate1H NMR (400 MHz, CDCl3) δ 6.84 (s, 2H), 3.79 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 165.4, 133.4, 52.3.
MS(EI): m/z (%): 144(1) [M]+, 113(100), 85(48), 59(18), 53(10).
2 Results and Discussion 2.1 Orthogonal experimentalIn order to evaluate the importance of different factors based on product yield of the dicarbonylation reaction, a series of orthogonal experiments were conducted where the following five variables were analyzed: reaction temperature (factor A), reaction time (factor B), partial pressure of air (factor C), partial pressure of carbon monoxide (factor D), KI/PaCl2 molar ratio (factor E). Four levels of each factor were investigated. The selected factors and levels were given in Table 1. Reference to the experimental design theory, the orthogonal array L16 (45) was selected to range the test program. The test results were listed in Table 2. Besides, a further orthogonal analysis was carried out, the K and R values were also calculated and presented in Table 2. R is defined as the range between the ma-ximum and minimum value of K1, K2, K3, K4 and is used for evaluating the importance of the factors, i.e. a larger R means a greater importance of the factor.
| Table 1 Levels and factors affecting the yield |
| Table 2 Orthogonal experimental and total yield |
It can be noted that the R value of factor C is closed to that of factor E, while they are much larger than that of factor A and B. The R value of factor D is the least among these five factors. Therefore, the effects of the five factors on the dicarbonylation of acetylene reaction followed the order: C≈E > A > B≈D. These results suggested that the amounts of air and KI in the catalytic system had an essential influence. Moreover, it can recombine the optimal reaction conditions (A4B3C4D3E4): reaction temperature was 70 ℃, reaction time was 6 h, partial pressure of air was 2.5 MPa, partial pressure of carbon monoxide was 1.6 MPa and KI/PdCl2 molar ratio was 9:1. The verification experiment was conducted with desired product yield up to 81.9%. Therefore we rationally confirm the optimal conditions with preliminary experiments, then single variable controlled experiments were conducted on further based on these preliminary results.
2.2 Effect of different cocatalystsFig.1 shows the effect of different cocatalysts on the total yield of dimethyl maleate and dimethyl fumarate. It can be observed that the cocatalyst KI was the most efficient among all the cocatalysts. The total yield reached 81.9%, which was 2 to 8 times larger than the other cocatalysts (KBr, FeCl3, CuCl2/HCl, FeCl3/H2SO4). The replacement of KI with KCl and KBr as cocatalyst gave almost no reaction. This is due to the difficulty of reoxidizing palladium (0) to palladium (Ⅱ) with air-KCl or air-KBr[1]. In contrast, I2 formed in situ with air-KI can reoxidize palladium(0) to palladium (Ⅱ) to regenerate the catalytic cycle. When CuCl2 and FeCl3 were employed as cocatalysts, the yield was very low. Even though an addition of acid had a great impact on catalytic behavior, the yield of CuCl2 and FeCl3 as cocatalyst were still much lower than KI. The possible reason is that air oxidizing I- anion to I2 is more efficient than oxidizing Cu+ to Cu2+ or Fe 2+ to Fe 3+. Besides, the "softer" binding nature of iodide compared with other halides could have some effects on the catalytic activity[15-16]. There, it is concluded that both redox properties under air atmosphere and coordinative capability play important roles in the catalytic performance for the dicarbonylation of acetylene with carbon monoxide to dimethyl maleate and fumarate.

|
Figure 1 Effect of different cocatalysts on the dicarbonylation of acetylene Conditions: PdCl2 0.056 mmol, cocatalyst 0.504 mmol, acetylene 16.13 mmol, methanol 30 mL, partial pressure: P(CO)=1.6 MPa, P(air)=2.5 MPa, time: 6 h, temperature: 70 ℃ a. concentrated hydrochloric acid 3.0 mL; b. concentrated sulfuric acid 1.1 mL |
Several co-solvents were used in this reaction and the results were presented in Table 3. The total yields were 87.5%, 77.0% and 51.0% with the co-solvents THF, methanol and dichloromethane, which were much higher than other co-solvents. This can be ascribed to the high solubility of acetylene and CO in them[17-18]. Moreover, the co-solvents acetone, DMF and DMSO can occupy the coordination sites with CO molecule[17], weaken the activation of CO molecular. And the previous works[17-18] reported that the reactivity pattern can also be affected by the polarity of the co-solvent. In addition, the polarity of the co-solvent has a pronounced effect on the product selectivity as the cis/trans isomer ratio increases with the polarity of the medium. However, solvents having high polarity such as acetone and DMF decreased the cis/trans isomer ratio, perhaps due to their strong capability for coordination toward palladium center.
| Table 3 Effect of co-solvents on the dicarbonylation of acetylene |
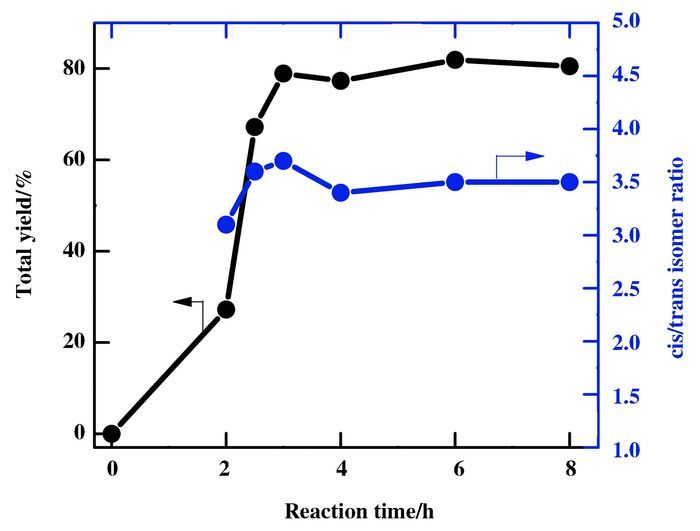
Fig. 2shows the effect of reaction time on the total yield and cis/trans isomer ratio. The total yield enhances drastically from 0% to 67.2% within 2.5 h. After 2.5 h, the total yield increases only a little. Subsequently, the total yield almost remains constant although the reaction time is lengthened which indicates acetylene is almost consumed. It is noteworthy that a nearly constant in the cis/trans isomer ratio is observed with reaction time. This indicates that isomerization of dimethyl maleate to its trans isomer, dimethyl fuma-rate, doesn't happen under the present conditions, even though trans isomer is thought to be more stable than its cis isomer, otherwise, the cis/trans isomer ratio should decrease with the increase of reaction time. In fact, the analogous result was found in palladium-catalyzed dicarbonylation of terminal alkyne to dicarboxylic acid[19].
|
Figure 2 Effect of reaction time on the dicarbonylation of acetylene Conditions: PdCl2 0.056 mmol, KI 0.504 mmol, C2H2 16.13 mmol, methanol 30 mL, partial pressure: P(CO)=1.6 MPa, P(air)=2.5 MPa, temperature: 70 ℃ |
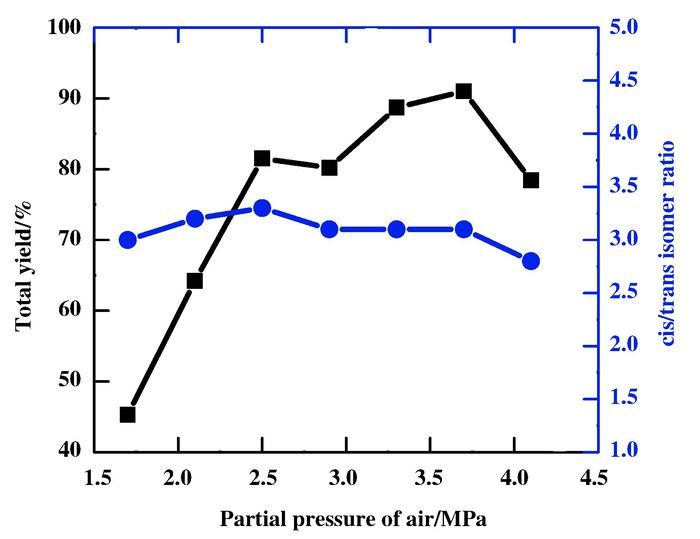
The effect of air pressure on the yield is shown in Fig. 3. As expected, air pressure has a strong influence on the total yield of dicarbonylation of acetylene. When initial partial pressure of air is 1.7 MPa, the yield is only 45.3%. Subsequently, the total yield increases significantly with increase in the partial pressure of air. In the scope of 2.5~3.7 MPa of the partial pressure of air, the yield increases slowly. Howe-ver, the yield decreases sharply with further increase of initial partial pressure of air up to 4.1 MPa. The results could be assigned to the appropriate air pressure contributes to oxidize I- anion to I2 which can reoxidize Pd(0) to Pd(Ⅱ), but excess of air can suppress the reduction of I2. The cis/trans isomer ratio remains almost constant with change in partial pressure of air, which perhaps due to that air containing N2 and O2 doesn't effect on the coordination of reactant to Pd centre.
|
Figure 3 Effect of partial pressure of air on the dicarbonylation of acetylene Conditions: PdCl2 0.056 mmol, KI 0.677 mmol, C2H2 16.13 mmol, methanol 30 mL, partial pressure: P(CO)=1.6 MPa, time: 3 h, temperature: 70 ℃ |
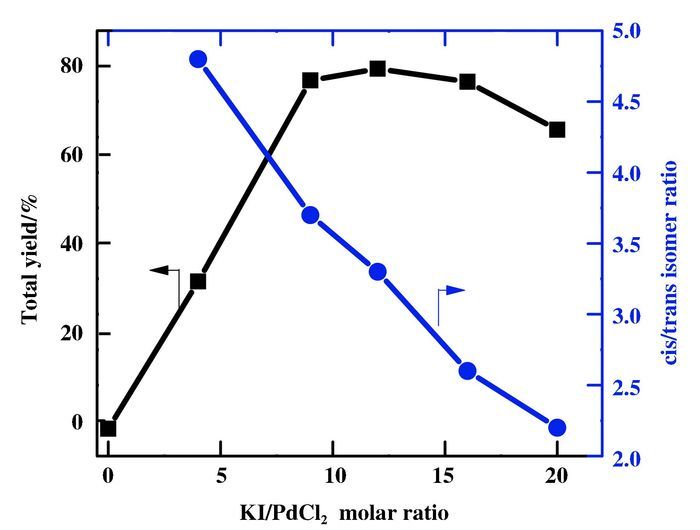
The molar ratio of KI/PdCl2 is also found to have a significant role in catalyst activity and product selectivity (Fig. 4). The catalytic activity increases up to a KI/PdCl2 molar ratio of twelve, and decreases steadily with further increase. High activity can be mainly ascribed to KI which can form I2 under the experimental conditions for reoxidizing Pd(0) to Pd(Ⅱ). With increase in KI/PdCl2 molar ratio, I- anion competes with the reactant molecules such as CO, acetylene for coordination toward Pd centre, causing decrease in the activity.
|
Figure 4 Effect of KI/PdCl2 molar ratio on the dicarbonylation of acetylene Conditions: PdCl2 0.056 mmol, C2H2 16.13 mmol, methanol 30 mL, partial pressure: P(CO)= MPa, P(air)=2.5 MPa, time: 3 h, temperature: 70 ℃ |
A gradual decrease in the cis/trans isomer ratio is observed with increase in KI/PdCl2 molar ratio. It is likely that the presence of I- anion contributes to the "trans" insertion of the coordinated acetylene, so that the intermediate complex B will be shifted toward intermediate complex D (Scheme 3), resulting the increased formation of the dimethyl fumarate.

|
Scheme 3 Plausible mechanism proposed for dicarbonylation of acetylene |
On the basis of the results, we proposed the following mechanisms (Scheme 3), which are similar to the possible mechanisms for palladium-catalyzed dicarbonylation of terminal and internal alkyne with carbon monoxide[19-23]. Firstly, coordination of CO and acetylene to palladium centre can form complex A. Nucleophilic attack of methanol on the coordinated CO would give the Pd-methoxycarbonyl species B after the elimination of HI and the incorporation of another CO. The next step is the insertion of the coordinated acetylene into the Pd-methoxycarbonyl bond giving vinyl-palladium complex C ("cis" insertion) or D ("trans" insertion). Complex C undergoes migratory insertion of CO into the palladium-vinyl bond to form complex E, followed by the nucleophilic attack of methanol and reductive elimination of HI to give Pd(0) and dimethyl maleate. Similarly, the vinyl-palladium complex D can undergo migratory insertion of CO into the palladium-vinyl bond to form complex F, followed by nucleophilic attack and reductive elimination to give dimethyl fumarate and Pd(0). The formed Pd(0) species can be reoxidized to Pd(Ⅱ) to regenerate the catalytic cycle by use of I2 as a reoxidant which is reduced to I- anion. Notably, the resulting I- anion is regenerated to I2 by air as the ultimate oxidant, making the process catalytic in both palladium and iodine.
3 ConclusionsDicarbonylation of acetylene has been studied using an acid-free catalytic system which contains PdCl2 and KI in the presence of CO and air. The orthogonal experiments demonstrated that the partial pressure of air and the amounts of KI are the crucial roles in the reaction process. Dicarbonylated products are mainly maleic esters and cis/trans isomer ratio is influenced significantly by the molar ratio of KI/PdCl2. A 91.0% yield and cis/trans isomer ratio 3.1:1 are obtained under the optimal experimental conditions.
| [1] | Gabriele B, Costa M, Salerno G, et al. An efficient and selective palladium-catalysed oxidative dicarbonylation of alkynes to alkyl-or aryl-maleic esters[J]. J Chem Soc Perkin Trans, 1994, 25(1): 83–87. |
| [2] | Müller S P, Kucher M, Ohlinger C, et al. Extrusion of Cu/ZnO catalysts for the single-stage gas-phase proces-sing of dimethyl maleate to tetrahydrofuran[J]. J Catal, 2003, 218(2): 419–426. DOI:10.1016/S0021-9517(03)00157-X |
| [3] | Mokhtar M, Ohlinger C, Schlander J H, et al. Hydrogenolysis of dimethyl maleate on Cu/ZnO/Al2O3 catalysts[J]. Chem Eng Technol, 2001, 24(4): 423–426. DOI:10.1002/(ISSN)1521-4125 |
| [4] | Chen L F, Guo P J, Zhu L J, et al. Preparation of Cu/SBA-15 catalysts by different methods for the hydrogeno-lysis of dimethyl maleate to 1, 4-butanediol[J]. Appl Catal A, 2009, 356(2): 129–136. DOI:10.1016/j.apcata.2008.12.029 |
| [5] | Schlander J H, Turek T. Gas-Phase Hydrogenolysis of dimethyl maleate to 1, 4-butanediol and γ-butyrolactone over copper/zinc oxide catalysts[J]. Ind Eng Chem Res, 1999, 38(4): 1264–1270. DOI:10.1021/ie980606k |
| [6] | Lan J, Chen Z, Lin J, et al. Catalytic aerobic oxidation of renewable furfural to maleic anhydride and furanone derivatives with their mechanistic studies[J]. Green Chem, 2014, 16(9): 4351–4358. DOI:10.1039/C4GC00829D |
| [7] | Wu X F, Neumann H, Beller M. Palladium-catalyzed oxidative carbonylation reactions[J]. Chem Sus Chem, 2013, 6(2): 229–241. DOI:10.1002/cssc.v6.2 |
| [8] | Shi Li-jun(石利军), Ren Ye-chao(任业超), Xia Chun-gu(夏春谷), et al. Carbonylation with the new nitrogen bidentate palladium catalysts(双齿氮配体钯配合物催化的羰化反应研究)[J]. J Mol Catal (China)(分子催化), 2011, 25(4): 289–294. |
| [9] | Liu Rui(刘蕊), Xiong Xu-mao(熊绪茂), Mu Xin-yuan(慕新元), et al. Study on the preparation of methyl acrylate by carbonylation of acetylene catalyzed with iron carbonyl(羰基铁催化乙炔羰化合成丙烯酸甲酯的研究)[J]. J Mol Catal (China)(分子催化), 2015, 29(2): 97–102. |
| [10] | Tsuji J, Morikawa M, Iwamoto N. Organic syntheses by means of noble metal compounds. Ⅵ. Synthesis of muconic acid[J]. J Am Chem Soc, 1964, 86(10): 2095–2095. |
| [11] | Chiusoli G P, Venturello C, Merzoni S. A novel synthesis of dimethyl maleate and dimethyl muconate[J]. Chem & Ind, 1968(29): 977. |
| [12] | Alper H, Despeyroux B, Woell J B. Selective hydroesterification of alkynes to mono-or diesters[J]. Tetrahedron Lett, 1983, 24(51): 5691–5694. DOI:10.1016/S0040-4039(00)94174-1 |
| [13] | Xu Song-yan(许松岩), Nie Zong-wu(聂宗武), Chen Yi-dun(陈贻盾), et al. Synthesis of dibutyl ester of butene diacid by the oxidative carbonylation of acetylene(乙炔氧化羰基化合成丁烯二酸二丁酯)[J]. Chin J Catal(催化学报), 1983, 4(1): 24–30. |
| [14] | Nie Zong-wu(聂宗武), Xu Song-yan(许松岩), Chen Yi-dun(陈贻盾), et al. On the kinetics and mechanism of the oxidative carbonylation of acetylene to dibutyl ester of butene diacid(乙炔氧化羰基化合成丁烯二酸二丁酯的反应动力学和机理)[J]. Chin J Catal(催化学报), 1985, 6(2): 149–154. |
| [15] | Bhanage B, Gadge S. Synthesis of α, β-alkynyl esters and unsymmetrical maleate esters catalyzed by Pd/C; An efficient phosphine-free catalytic system for oxidative alkoxycarbonylation of terminal alkynes[J]. Synlett, 2013, 24(08): 981–986. DOI:10.1055/s-00000083 |
| [16] | Maitlis P M, Haynes A, James B R, et al. Iodide effects in transition metal catalyzed reactions[J]. Dalton Trans, 2004(21): 3409–3419. DOI:10.1039/b410128f |
| [17] | Seayad A, Kelkar A A, Toniolo L, et al. Hydroesterification of styrene using an in situ formed Pd(OTs)2(PPh3)2 complex catalyst[J]. J Mol Catal A, 2000, 151(1): 47–59. |
| [18] | Shiho T, Masanori M, Hideki K, et al. Selective mono-and bis(alkoxycarbonylation)s of olefins catalyzed by palladium in the presence of Cu(I) or Cu(Ⅱ) chloride under remarkably mild conditions. Application to the synthesis of γ-butyrolactone derivatives[J]. Bull Chem Soc Jpn, 1991, 64(12): 3600–3606. DOI:10.1246/bcsj.64.3600 |
| [19] | Zargarian D, Alper H. Palladium chloride catalyzed dicarbonylation of terminal alkynes[J]. Organometallics, 1991, 10(8): 2914–2921. DOI:10.1021/om00054a070 |
| [20] | Heck R F. Dicarboalkoxylation of olefins and acetylenes[J]. J Am Chem Soc, 1972, 94(8): 2712–2716. DOI:10.1021/ja00763a028 |
| [21] | Cassar L, Chiusoli G, Guerrieri F. Synthesis of carboxy-lic acids and esters by carbonylation reactions at atmospheric pressure using transition metal catalysts[J]. Synthesis, 1973, 1973(09): 509–523. DOI:10.1055/s-1973-22246 |
| [22] | Beltrani M, Carfagna C, Milani B, et al. Oxidative alkoxycarbonylation of alkynes by means of aryl α-diimine palladium(Ⅱ) complexes as catalysts[J]. Adv Synth Catal, 2016, 358(20): 3244–3253. DOI:10.1002/adsc.201600521 |
| [23] | Giannoccaro P, Aresta M, Doronzo S, et al. Phenylace-tylene carbonylation catalysed by Pd (Ⅱ) and Rh (Ⅲ) intercalated in zirconium phosphates[J]. Appl Organomet Chem, 2000, 14(10): 581–589. DOI:10.1002/(ISSN)1099-0739 |
 2017, Vol. 31
2017, Vol. 31


