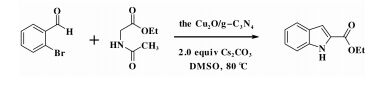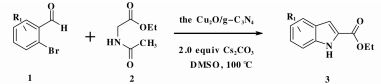2. College of Chemistry and Chemical Engineering, China West Normal University, Nan Chong 637002, China
2. 西华师范大学 化学化工学院, 四川, 南充 637002
The indole nucleus, recognized as a biologically significant moieties[1-4], has been the target of numerous synthetic methods[5-9]. Often, synthetic strategies introduced copper compounds to catalyze the indole derivatives formation reaction in excellent yields[10-12], and proceeded via a presumed mechanism (Scheme 1). Unfortunately, many of these transformations applied to the indole ring system need a high dosage of copper catalyst, give merely moderate yields (34%~53%)[10-11] and are not favorable for the sufficient se-paration of catalysts and/or products. Overall, it would not be most welcome from atom-economy and environment perspective.

|
Scheme 1 The plausible mechanism of indole formation |
Graphitic carbon nitride (g-C3N4) as a sort of polymeric super molecular materials exhibits excellent stability as well as good environmental acceptance. The surface of g-C3N4 contains a small amount of hydrogen present as primary and/or secondary amine groups on the terminating edges, which can form a quantity of surface defects along with its unique dimensional layered structure. It is favorable for hybridizing with other components, due to this structure fragment and the electronic properties (a larger size of the π system). Accordingly, g-C3N4 features almost the precursor of transition metal heterogeneous catalyst[13-16]. Herein, we would like to synthesize the Cu2O/g-C3N4 on the pioneering work of predecessor, and utilize it to catalyze the formation of indole-2-carboxylic esters in order to avoid catalyst's separation problems, the low yields and the high loading of catalyst, starting from 2-bromobenzaldehyde and ethyl acetamidoacetate.
1 Experimental section 1.1 Preparation of the g-C3N4 polymeric materialsFirstly, melamine (2.5 g) powder was put into a semi-closed alumina crucible with a cover under N2 gas flow to 550 ℃ for 4 h at a heating rate of 5 ℃/min, followed by naturally cooling to room temperature. After cooling, the yellow product was milled and collected.
1.2 Preparation of the Cu2O/g-C3N4 composite0.2 g of g-C3N4 and 0.075 g of CuCl2·2H2O were ultrasonically dispersed in 100 mL mixture solution of H2O/EtOH (the volume rate is 1:1) for 1.5 h, and 100 mL of aqueous solution containing 0.165 8 g of NaBH4 was added dropwise under stirring at 25 ℃. The yellow-brown solution changed gradually to deep blue within 0.5 h. Then the deep blue solution was stirring for 16 h. The product were centrifuged and washed with distilled water and EtOH for several times, and dried in a vacuum oven at 60 ℃. The brown product was obtained in 0.23 g.
1.3 CharacterizationThe X-ray diffraction patterns of the Cu2O/g-C3N4 and the g-C3N4 were recorded by diffractometer (D/MAX Ultima Ⅳ, Rigaku, Japan). The X-ray photoelectron spectroscopy spectra were examined on the X-ray energy spectromer (Kratos Analytical Axis Ultra, United Kingdom). Fourier transform infrared spectroscopy spectra were scanned on the Nicolet-6700 spectrometer. SEM measurements were carried out by the JSM-6510LV scanning electron microscopy (Rigaku, Japan).
1.4 General procedure for indole-2-carboxylic estersCaesium carbonate (652 mg, 2 mmol) and catalyst (30 mg) were added to a dried reaction tube. The tube was filled with nitrogen before DMSO (2 mL) was added. Then 2-bromobenzaldehyde (185 mg, 1 mmol) and ethyl acetamidoacetate (156.2 mg, 1.2 mmol) were added prior to heating the slurry to 100 ℃ under a nitrogen atmosphere for 12 h with good agitation. After cooled down, the mixture was added DI water (5 mL) and extracted with ethyl acetate (5 mL) for 3 times. The combined organic fraction was washed with DI water (5 mL) and brine (5 mL). After dried over sodium sulfate and filtered, the organic phase was concentrated to give crude product. The crude product was further analyzed by GC Agilent 7890A gas chromatography using HP-5 column (30 m × 0.32 mm × 0.25 μm) and FID detector
2 Results and DiscussionSchematic illustration of the synthesis process of the Cu2O/g-C3N4 composite is indicated in Scheme 2. The first step requires the calcination of melamine at high temperature to obtain the entitled product (g-C3N4). The second step is the adsorbing of Cu2+ on the surface defects of g-C3N4 with the addition of CuCl2·2H2O. The third step is the reduction reaction of Cu2+ under the presence of reducing agent.
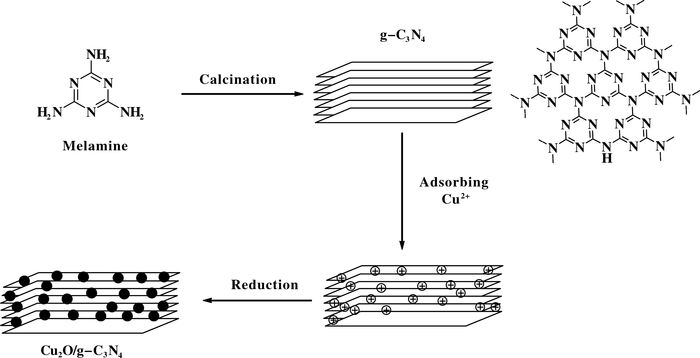
|
Scheme 2 Illustration of the preparation of Cu2O/g-C3N4 |
The actual images of the Cu2O/g-C3N4 and g-C3N4 are exposed in Fig. 1. The g-C3N4 is off-white powder. Compared to g-C3N4, the Cu2O/g-C3N4 is a small fulvous block-liker, which suggests that Cu2O is successfully adsorbed on the surface defects of g-C3N4 from the facade of the prepared product.

|
Figure 1 The digital photographs of the Cu2O/g-C3N4 and g-C3N4 |
As shown in Fig. 2, the FTIR spectrum of the Cu2O/g-C3N4 is similar with that of the g-C3N4, because of no characteristic peak of Cu2O. The peak at 810 cm-1 is attributed to out of bending modes C-N heterocycles, while the peak between 1 250 and 1 650 cm-1 are assigned to aromatic C=N and C-N stretching vibration. The measurement result is correspond to the reported literature[17].
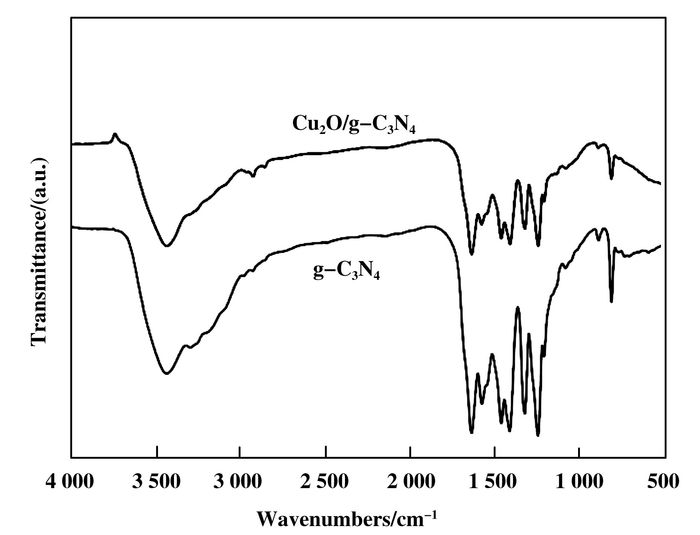
|
Figure 2 Fourier transform infrared spectroscopy of the Cu2O/g-C3N4 and g-C3N4 |
XRD and XPS analysis were performed to further confirm the formation of the Cu2O/g-C3N4. The full range XPS spectrum of Cu2O/g-C3N4 (Fig. 3) reveals that the composite contains C, N, O, and Cu element. It was clear that the peak appearing at 530.6 eV implied the presence of O2- and the peak at 287.9 eV was attributed to the carbon of C—C, while the characteristic peak at 932.6, 952.5 eV were assigned to Cu+[18-20].
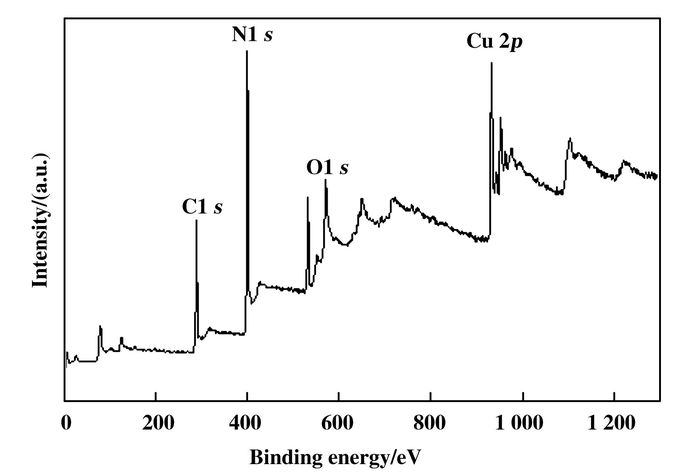
|
Figure 3 The full range XPS spectrum of Cu2O/g-C3N4 |
The diffraction peaks of Cu2O/g-C3N4, Cu2O and pure Cu can be observed simultaneously, according to the information provided by the schematic diagram (Fig. 4). The peak's intensity (at 42.2°, 61.6°, 73.6°) of pure Cu is much weaker than that (at 37.7°) of Cu2O, which reveals the presence of a light amount of pure Cu. The high intensity peak located at 27.6° is corresponded to the pure g-C3N4, which is in accordance with previous report[21].
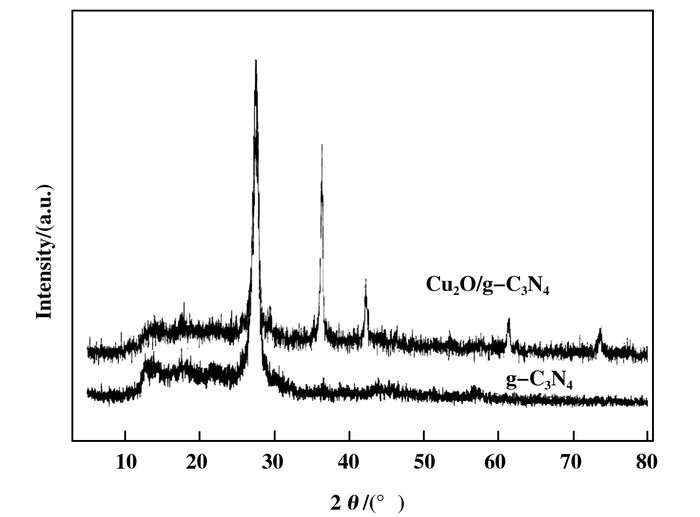
|
Figure 4 The XRD spectra of Cu2O/g-C3N4 and g-C3N4 |
Additionally, SEM measurement was performed to investigate the microstructure of the Cu2O/g-C3N4 and g-C3N4. From the SEM image (Fig. 5A) of g-C3N4, it can be seen that the g-C3N4 appears the sheet spatial structure and bending aggregation. It is visible from Fig. 5B that the appearance characteristics of g-C3N4 are modified, which is caused by the adsorption of numerous Cu2O. All of these measurement results can confirm that the prepared sample is the Cu2O/g-C3N4 composite though there is a trace amount of pure copper.
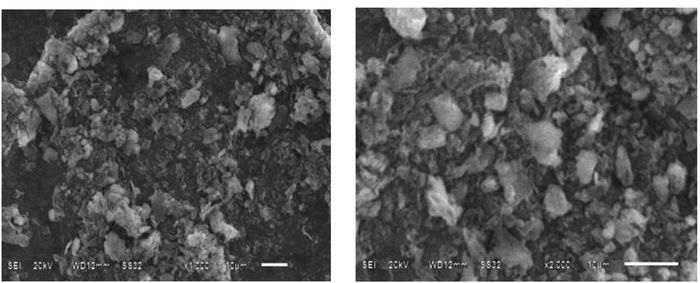
|
Figure 5 SEM image A of the g-C3N4 and SEM image B of the Cu2O/g-C3N4 |
During the course of work, we set out to research the catalytic potentiality of Cu2O/g-C3N4 for the synthesis of indole-2-carboxylic esters, using 2-bromobenzaldehyde and ethyl acetamidoacetate as reaction substrates in DMSO. To begin with, what is investigated that the catalytic efficiency of this transformation is affected by the embedded quantity of Cu in the case of the Cu2O/g-C3N4 particles (see Table 1).
| Table 1 Screening of condition with the loading of Cu in the case of the Cu2O/g-C3N4a |
A low yield of 7.70% and 13.5% was obtained when the reaction was proceeded with the pure g-C3N4 and Cu2O respectively (Table 1, entry 5 and 6). Meanwhile, the mixture of Cu2O and Cu gave the yield of 8.20% (Table 1, entry 7). As anticipated, the catalytic activity of this reaction was improving as the loading of Cu increasing until 12.3% in the case of the Cu2O/g-C3N4 (Table 2, entries 1-4). So, the Cu loading (12.3%) of the Cu2O/g-C3N4 was selected as the heterogeneous catalyst in the following investigation. After examining of the effects of various embedded amount of Cu, various bases and reaction media were explored. Following the bases and solvents screen (Table 2), we found no reaction took place at all using 1-decanol as solvent (Table 2, entry 7). Meanwhile, a trace amount of the desired product was observed in the DMF which was proven to be preferable solvent for this reaction[10]. Other solvents such as NMP, Toluene, 1, 4-dioxane failed to provide more than a trace amount of the desired products (Table 2, entries 2-4). To our delight, the yield still resulted in 35.9% and 31.5% respectively when DMSO and Ethyl acetate were employed as the solvent (Table 2, entries 1 and 5). Subsequently, the influence of the base was evaluated in DMSO. A series of base (K2CO3, K3PO4, CH3COONa, LiOH, KOH, NaOH, KHCO3) were investigated in the reaction, and we found that the screened base (Cs2CO3), giving good yield (35.9%), was preferred for this transformation (Table 2, entry 1 vs entries 8-14). A slight decrease in the amount of Cs2CO3 resulted in an obvious decrease in the yield of the desired product (Table 2, entry 15). Considering the moderate basicity, we chose Cs2CO3 (2 equiv) as base in the formation of indole-2-carboxylic esters.
| Table 2 Probing condition of base and solvent for this transformationa |
Furthermore, we attempted to investigate the influence of reaction temperature and the catalyst loading (the Cu2O/g-C3N4) in the transformation. As seen in Table 3, the yields were found to increase with a raising in the temperature (Table 3, entries 1-3) although the condition of high temperature (120 ℃) retarded the reaction (Table 3, entry 4). In the case of catalyst (the Cu2O/g-C3N4) dosage, the experiments performed similarly with that of the temperature: increasing the dosage of the Cu2O/g-C3N4 composite improved the yield of the reaction (Table 3, entries 3, 5-6). Surprisingly, the high dosage (40 mg) of catalyst led to a slight decrease in the yield (Table 3, entry 7). From what has been discussed above, the optimal condition of the one-pot three-step formation of indole-2-carboxylic esters is refined as follow: the Cu2O/g-C3N4 (30 mg, 5.8% Cu), Cs2CO3 (2.0 equiv), DMSO (2 mL) and 100 ℃.
| Table 3 Optimizingreaction temperature and the amount of catalysta |
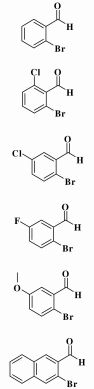
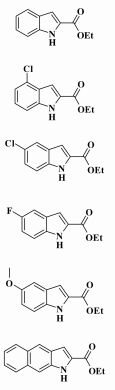
With our reaction conditions refined in hand, we further probed the scope of this novel methodology. Thus, a series of starting substrates were subjected to the reaction in DMSO (Table 4). All of benzaldehydes containing electron-rich groups reacted to give the expected indole product in the range of 30.6%~34.4% yield (Table 4, entries 5 and 6), while electron-poor substrates did not react well with this system (Table 4, enties 2-4).
| Table 4 Synthesis of Various Heterocycle-2-carboxylic Estersa |
In summary, the Cu2O/g-C3N4 composite was synthesized by the calcination of melamine, ultrasonic exfoliation, and reduction of Cu2+ adsorbed on the defects of g-C3N4, and characterized by FTIR, XRD, XPS and SEM. Meanwhile, the Cu2O/g-C3N4 was utilized to catalyze the formation of indole-2-carboxylic esters and gave desired yield although the low dosage (30 mg, 5.8% Cu) of catalyst was used. To the best of our knowledge, this procedure has not been reported to date.
| [1] | Biersack B, Schobert R. Indole compounds against breast canser:Recent development[J]. Curr Drug Targets, 2012, 13(14): 1705–1719. DOI:10.2174/138945012804545551 |
| [2] | Kaushik N K, Attri P, Kumar N, et al. Biomedical importance of indoles[J]. Molecules, 2013, 18(6): 6620–6662. DOI:10.3390/molecules18066620 |
| [3] | Brewer M R, Pao W. Maximizing the benefits of off-target kinase inhibitor activity[J]. Cancer Discov, 2013, 3(2): 138–140. DOI:10.1158/2159-8290.CD-12-0581 |
| [4] | Ahmad A, Sakr W A, Rahman K M. Mechanisms and therapeutic implications of cell death induction by indole compounds[J]. Cancers, 2011, 3(3): 2955–2974. |
| [5] | Inman M, Moody C J. Indole synthesis-Something old, something new[J]. Chem Sci, 2013, 4(1): 29–41. DOI:10.1039/C2SC21185H |
| [6] | Shi Z, Glorious F. Efficient and versatile synthesis of indoles from enamines and imines by cross-dehydrogenative coupling[J]. Angew Chem Int Ed, 2012, 51(37): 9220–9222. DOI:10.1002/anie.201205079 |
| [7] | Taber D F, Tirunahari P K. Indole synthesis:A review and proposed classification[J]. Tetrahehdron, 2011, 67(38): 7195–7210. DOI:10.1016/j.tet.2011.06.040 |
| [8] | Cacchi S, Fabrizi G, Goggiamani A. Indoles via palladium catalyzed cyclization[J]. Org Rea, 2012, 76(4): 281–534. |
| [9] | McNulty J, Keskar K. A tandem "on-palladium" Heck-Jeffery amination route toward the synthesis of functiona-lized indole-2-carboxylates[J]. Eur J Org Chem, 2011, 34(34): 6902–6908. |
| [10] | Stefan G K, John W D, Yan B L, et al. A ligand-free, copper-catalyzed cascade sequence to indole-2-carboxylic esters[J]. Tetrah, 2010, 51(50): 6549–6551. DOI:10.1016/j.tetlet.2010.10.035 |
| [11] | Stefan G K, John W D, Yan B L, et al. Copper-Catalyzed synthesis of indoles and related heterocycles in renewable solvents[J]. ACS Sust Chem Eng, 2014, 2(6): 1359–1363. DOI:10.1021/sc5002098 |
| [12] | David S S, Stephen L B. Diamine ligands in copper-catalyzed reactions[J]. Chem Sci, 2010, 1(1): 13–31. DOI:10.1039/c0sc00107d |
| [13] |
a. Zhu H, Chen X, Zhao J C, et al. Mechanism of supported gold nanoparticles as photocatalysts under ultraviolet and visible light irradiation[J]. Chem Commun, 2009, 48(48): 7524-7526. b. He Ping(何平), Chen Yong(陈勇), Fu Wen-fu(傅文甫). Study of visible-light driven preparation of Fe/g-C3N4 composite catalyst with simultaneous hydrogen evolution(可见光驱动制备Fe/g-C3N4复合催化剂及其产氢研究)[J]. J Mol Catal(China)(分子催化), 2016, 30(3): 269-275. c. Ma Lin(马琳), Kang Xiao-xue(康晓雪), Hu Shao-zheng(胡绍争), et al. Preparation of Fe, P Co-dopedGraphitic carbon nitride with enhanced visible-light photocatalytic activity(Fe-P共掺杂石墨相氮化碳催化剂可见光下催化性能研究)[J]. J Mol Catal(China)(分子催化), 2015, 29(4): 359-368. |
| [14] | Vien V, Nguyen V K, Sung J K, et al. Preparation of g-C3N4/Ta2O5composites with enhanced visible light photocatalytic activity[J]. Elect Mater, 2016, 45(5): 2334–2340. DOI:10.1007/s11664-015-4280-9 |
| [15] | Yong C B, Ke Z C. AgCl/Ag/g-C3N4 hybrid compo-sites:preparation, visible light-driven photo catalytic activity and mechanism[J]. Nano Micro Lett, 2016, 8(2): 182–192. DOI:10.1007/s40820-015-0076-y |
| [16] | Yasir A, Qun H, Dao B L, et al. Facile synthesis of mesoporous detonation nanodiamond-modified layers of graphitic carbon nitride as photocatalysts for the hydrogen evolution reaction[J]. RSC Adv, 2017, 7(25): 15390–15396. DOI:10.1039/C7RA02178J |
| [17] | Ge L. Synthesis and photocatalyic performance of novel metal-free g-C3N4 photocatalyst[J]. Mater Lett, 2011, 65(17/18): 2652–2654. |
| [18] | Zou S W, How C W, Chen J P. Photocatalytic treatment of wastewater contaminated with organic waste and copper ions from the semiconductor industry[J]. Ind Eng Chem Res, 2007, 46(2): 6566–6571. |
| [19] | Spasiano D, Rodriguez L D P, Olleros J C, et al. TiO2/Cu(Ⅱ) photocatalytic production of benzaldehyde from benzyl alcohol in solar pilot plant reactor[J]. Appl Catal B, 2013, 56(5): 136–137. |
| [20] | Chusshuei C C, Brookshier M A, Goodman D W. Correlation of relative X-way photoelectron spectroscopy shake-up intensity with CuO partcile size[J]. Langmuir, 1999, 15(8): 2806. DOI:10.1021/la9815446 |
| [21] | X Wang, K Maeda, A Thomas, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light[J]. Nat Mater, 2009, 8(1): 76–80. DOI:10.1038/nmat2317 |
 2017, Vol. 31
2017, Vol. 31