2. 昆明理工大学 环境科学与工程学院, 云南 昆明 650500;
3. 云南省高校磷化工重点实验室, 云南 昆明 650500
2. Faculty of Environmental Science and Engineering, Kunming University of Science and Technology, Kunming 650500, China;
3. The Higher Educational Key Laboratory for Phosphorus Chemical Engineering of Yunnan Province, Kunming 650500, China
近年来, 随着全球丙烯需求量的日益增长, 石油资源日渐短缺, 价格与日攀升, 依靠传统的催化裂化和石脑油裂解副产丙烯已经满足不了化工生产对丙烯的需求, 开发新一代的丙烯生产工艺已成为石化行业的主要发展趋势[1].我国低碳烷烃资源比较丰富, 尤其是随着我国页岩气开采技术的发展, 丙烷催化脱氢逐渐成为提高丙烯产量的有效途径[2].丙烷脱氢制丙烯不但可以提高产品的附加值, 减少丙烯生产对石脑油裂解及催化裂化工艺的依赖程度, 而且还可以副产高价值的氢气[3].丙烷脱氢作为增加丙烯产量的重要途径而备受关注和重视.
目前, PDH丙烷脱氢主要有两种类型, 包括氧化脱氢(OPDH)和非氧化脱氢(PDH). OPDH是以丙烷与氧化剂(O2、CO2和NO等)[4-5]为原料来进行氧化脱氢的反应, 其催化反应机理不明确且技术尚不成熟[3].特别是氧化脱氢因有氧化剂的存在, 易造成反应深度氧化, 使产物选择性降低[4, 6]. PDH是采用丙烷为原料来直接进行催化脱氢制丙烯的工艺, 因其具有安全性、高丙烯回收率、应用性强等优势而受到广泛关注[7].所以研究一种高效、廉价的催化剂成为丙烷脱氢制丙烯生产工艺的重点.
Cr基催化剂在丙烷脱氢反应中性能优良, 与贵金属相比, 其对原料中杂质的要求低, 且价格低廉.目前Cr基催化剂的研究主要集中在活性组分铬的负载量[9]、铬物种的价态[10]以及活性组分铬与不同载体之间的相互作用[11].值得注意的是, 在铬基催化剂研究中如何获得高分散Cr, 特别是活性组分Cr的分散情况是铬基催化剂研究的另一个重点.典型的介孔分子筛MCM-41具有均匀且有序的介孔通道, 其孔径可控制在2~10 nm, 表面积高(约1000 m2 ·g-1)[7], 因此被广泛用作催化剂载体.虽然已有文献报道了Cr/MCM-41催化剂在异丁烷、乙烷脱氢和OPDH的应用[7, 12-15].但关于Cr/MCM-41催化剂在PDH的文献研究较少.因此, 将Cr/MCM-41催化剂用于PDH具有一定的研究意义.我们分别采用连续吸附法和传统浸渍法制备了Cr/MCM-41催化剂(分别记为Cr/MCM-41-ad和Cr/MCM-41-imp), 将制备的Cr/MCM-41催化剂用于PDH反应, 并对反应过程中温度, 空速, 压力等反应条件进行优化, 同时在合适的条件下开展两种不同方法制备的催化剂脱氢性能的研究.实验中采用XRD、SEM-mapping、UV-Vis、XPS、H2-TPR等表征手段对催化剂进行表征分析, 在此基础上比较分析了Cr/MCM-41-ad和Cr/MCM-41-imp两种方法下制备的催化剂脱氢性能的差异.
1 实验部分 1.1 实验试剂十六烷基三甲基溴化铵(CATB), NH3·H2O (25%(重量百分比)NH3), 正硅酸四乙脂(TEOS), (NH4)2 CrO4, 去离子水.实验所用试剂均为分析纯.
1.2 载体制备量取635.5 mL去离子水和14 g CTAB放于烧杯中搅拌20 min, 加入54.5 mL NH3·H2O继续搅拌, 待搅拌均匀后量取57.6 mL TEOS缓慢滴加到烧杯中, 持续搅拌1 h, 然后微波加热5 min, 取出冷却, 抽滤.于105 ℃烘箱中干燥24 h.未焙烧样品为含CTAB的SiO2材料(吸附剂), 焙烧去掉CTAB的样品为MCM-41载体.
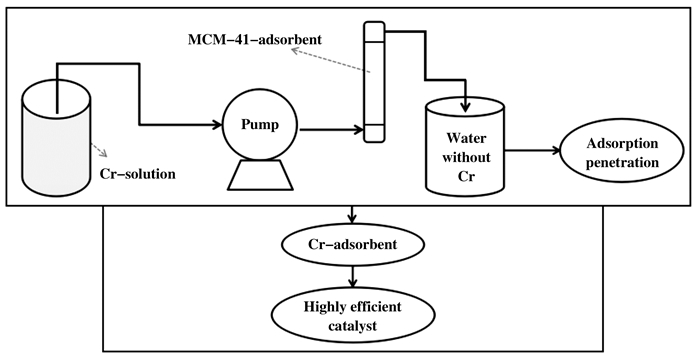
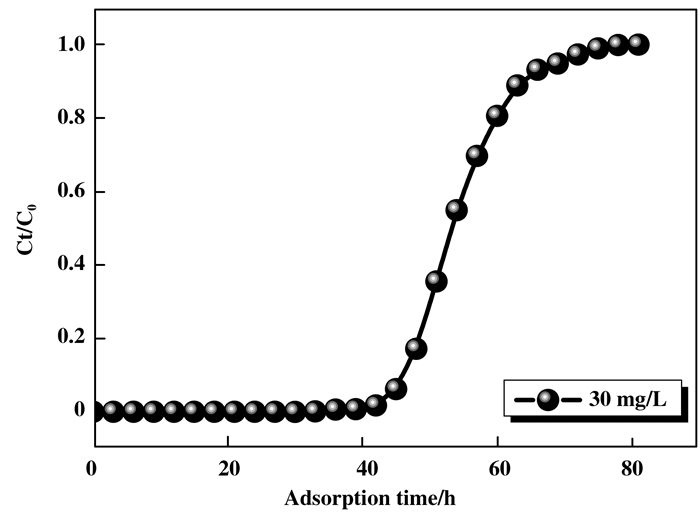
1.3 催化剂的制备 1.3.1 连续吸附法(adsorption)用K2Cr2O7制备含有30 mg / L Cr(Ⅵ)的溶液, 并使用HCl和NaOH调节至pH = 2.0.然后, 将一定量的制备的吸附剂(未煅烧)装入玻璃柱固定床(玻璃柱的长度和内径分别为200和11 mm), 以确保床高度为70 mm, 吸附过程流程如图 1.蠕动泵通过蠕动泵将含Cr(Ⅵ)的流入物以2.2 mL / min的流速从容器引导至固定床, 直至其饱和.通过原子吸收光谱法(Agilent AAS 240)测定Cr(Ⅵ)浓度.取出吸附材料, 静置10 h, 于90 ℃干燥箱中干燥6 h.随后放入马弗炉中焙烧5 h.由图 2吸附穿透曲线可以看出, 在吸附完成时出口浓度与进口浓度比值为1, 说明吸附柱中的吸附剂已吸附饱和.制备出的催化剂命名为Cr/MCM-41-ad.通过XRF表征手段可知Cr/MCM-41-ad的Cr负载量为13.5%.
|
图 1 连续吸附法流程图 Figure 1 Flow chart of adsorption column |
|
图 2 吸附穿透曲线 Figure 2 Adsorption breakthrough curve |
采用等体积浸渍法制备负载量为15%(重量百分比)的催化剂, 首先测得MCM-41的浸水率为5 mL水对应1 g载体.称取定量的前驱体(NH4)2CrO4放入坩埚中, 量取10 mL水于坩埚中搅拌至前驱体完全溶解, 加入2 g MCM- 41继续搅拌一段时间.室温下静置10 h, 于90 ℃干燥箱中干燥6 h.随后放入马弗炉中焙烧5 h.制备出的催化剂命名为Cr/MCM-41-imp.
1.4 催化剂的表征XRD用于催化剂的晶型结构测定, 采用X射线粉末衍射仪(XRD, PANalytical’s Empyren)对催化剂的结构进行表征.扫描电子显微镜(SEM)分析采用日本日立冷SU8220型场发射扫描电镜. X射线光电子能谱(XPS)通过PHI 5000反探针Ⅱ光谱仪测定Cr元素价态, 以C 1s的结合能284.6 eV为标准校正荷电子效应, CasaXPS处理软件用于背景消除及峰拟合. UV-Vis采用TU-19型双光束紫外可见分光光度计进行测定, 以BaSO4作为参比, 扫描的波长范围为200~800 nm. H2-TPR用于研究催化剂氧化还原性能.取100 mg(0.450~0.280 mm)催化剂置于石英玻璃管, 在100 ℃下通入氢气(90%Ar/10%H2)以10 ℃/min速率升温至800 ℃, 以TCD为检测器进行检测.
2 结果与讨论 2.1 条件优化反应条件作为丙烷催化脱氢过程中一个不可忽视的因素, 对催化剂性能有着重要的影响.在丙烷脱氢反应过程中, 反应温度、反应空速、反应压力等均对Cr/MCM-41催化剂的丙烷脱氢性能产生影响.以Cr/MCM-41-ad催化剂进行丙烷脱氢反应的条件优化实验.
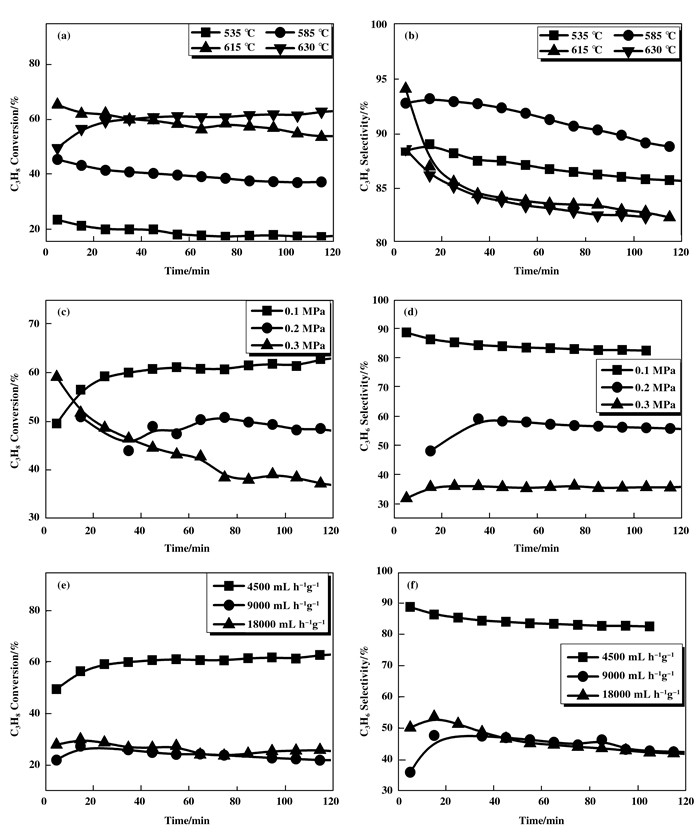
为考查温度对Cr/MCM-41-ad催化剂性能的影响, 分别选择了535、585、615和630 ℃对催化剂进行评价, 实验以0.4 g催化剂进行, 此时丙烷的体积空速为4500 mL·h-1·g-1, 压力为0.1 MPa.图 3a所示, 丙烷转化率随温度升高而上升, 反应温度越高, 丙烷转化率越大, 反应温度在630 ℃时丙烷转化率最高, 达到63%左右.同时考察温度对丙烯选择性的影响, 从图 3b可见, 在585 ℃时丙烯的选择性最高, 但此时丙烷的转化率太低, 仅为40%左右, 在615和630 ℃时丙烯选择性基本接近, 约为83%左右.综合考虑, 反应的合适的温度为630 ℃左右.
|
图 3 反应条件对催化性能影响 Figure 3 Effects of reaction conditions on catalytic performance (a) and (b) are temperatures; (c) and (d) are pressures; (e) and (f) are space velocities |
在反应温度为630 ℃、反应空速为4500 mL·h-1·g-1条件下, 考察了反应压力对Cr/MCM-41-ad催化能的影响, 实验以0.4 g催化剂进行.反应实验结果如图 3(c)和(d)所示, 随着反应压力的增大, 丙烷转化率和丙烯选择性均下降.当压力为0.1 MPa时逐渐稳定在63%左右, 此时, 丙烯的选择性达到最大值, 稳定在85%左右.当压力为0.2 MPa时, 丙烷的转化率稳定在50%左右, 但此时的选择性为60%左右.当压力为0.3 MPa时, 丙烷的转化率稳定在40%左右, 丙烯的选择性稳定在35%左右, 均为最低.综合考虑, 本实验的合适的反应压力为0.1 MPa.
在反应温度为630 ℃、反应压力为0.1 MPa的条件下, 考察了丙烷的体积空速对Cr/MCM-41-ad催化剂性能的影响.实验固定催化剂为0.2 g, 改变反应气流速: 15、30和60 mL/min来测定空速对选择性的影响.实验结果如图 3 (e)和(f)所示, 体积空速对转化率和选择性均有较大的影响, 空速越低转化率越高.空速为4500 mL·h-1·g-1时, 丙烷转化率稳定在58%左右, 转化率最高.空速为9000 mL·h-1·g-1和18 000 mL·h-1·g-1时转化率降低.由图 3 (f)可以看出选择性随空速的增加而升高, 但受限于丙烷脱氢反应动力学的限制, 丙烯收率出现下降.空速为9000 mL·h-1·g-1和18 000 mL·h-1·g-1的转化率均稳定在31%左右, 这是因为当空速高于9000 mL·h-1·g-1时丙烷转化率不受空速影响, 表明没有外扩散的影响, 使得转化率趋于稳定[15].综合考虑, 本实验合适的的反应空速为4500 mL·h-1·g-1.
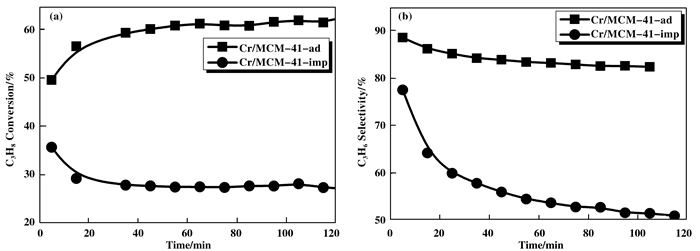
2.2 两种不同制备方法的催化剂活性的比较在催化剂为0.4 g、温度为630 ℃、空速为4500 mL·h-1·g-1、压力为0.1 MP条件下考察了两种不同方法制得的催化剂Cr/MCM-41在丙烷脱氢反应中催化剂活性的比较.实验结果如图 4所示, 在合适的反应条件下, Cr/MCM-41-ad的丙烷转化率达到了63%左右, 同时丙烯选择性同样稳定在85%左右, 而Cr/MCM-41-imp的丙烷转化率仅有30%左右, 同时丙烯选择性稳定在55%左右, 所以Cr/MCM-41-ad在PDH的催化性能与Cr/MCM-41-imp相比, 具有明显的优势. Cr/MCM-41-imp催化剂的活性随反应时间的增加而降低, 而Cr/MCM-41-ad的活性在120 min内保持稳定.通过对反应后的Cr/MCM-41-imp和Cr/MCM-41-ad催化剂进行拉曼的表征, 拉曼光谱在1200~1700 cm-1处有两个积碳峰, 对比发现Cr/MCM-41-imp的积碳峰高于Cr/MCM-41-ad, 说明Cr/MCM-41-imp催化剂在反应中产生的积碳多余Cr/MCM-41-ad.考虑到催化剂的失活再生情况, 对反应后的催化剂在空气下经过两次失活再生测验, 发现Cr/MCM-41-ad催化活性下降的并不是特别明显.说明吸附法负载Cr后的催化剂具有比较好的失活再生性能.
|
图 4 Cr/MCM-41-ad和Cr/MCM-41-imp催化剂的转化率(a)和选择性(b) Figure 4 Conversion of catalysts (a) and selectivity (b) for two different preparation methods |
Cr/MCM-41-ad与Cr/MCM-41-imp的XRD谱图如图 5所示.在(2θ=24.5°, 33.74°, 36.1°, 50.84°, 54.6°, 64.04°, 65.8°)出现的衍射峰, 归结为α-Cr2O3晶相的特征峰, 没有出现CrO3的衍射峰[13, 16-17].且吸附法负载Cr后的比表面积(1004.619 m2/g)高于浸渍法(925.147 m2/g).结果说明, CrO3在Cr/MCM-41-ad上的分散性可能比较好, 而Cr/MCM-41-ad的衍射峰明显小于Cr/MCM-41-imp的衍射峰, 表明在Cr/MCM-41-imp上形成大量的α-Cr2O3使其比表面积下降.经过计算Cr/MCM-41-ad和Cr/MCM-41-imp上α-Cr2O3晶体粒径列于表 1, 说明Cr/MCM-41-ad上α-Cr2O3晶体粒径小于Cr/MCM-41-imp.
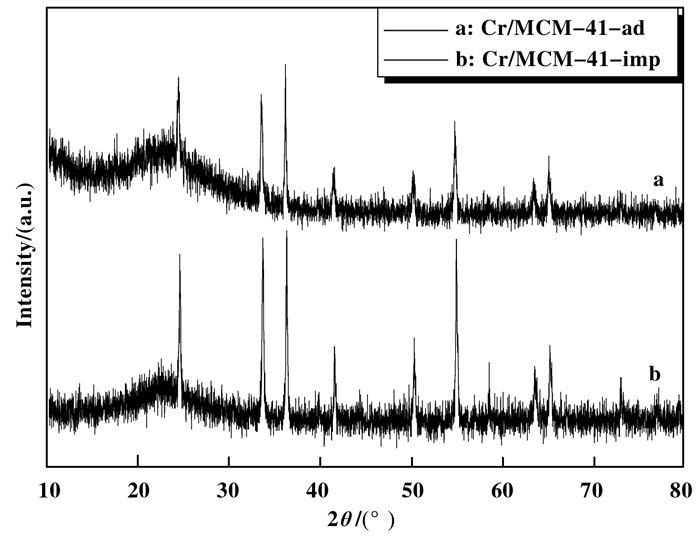
|
图 5 Cr/MCM-41-ad和Cr/MCM-41-imp的XRD图 Figure 5 Cr/MCM-41-adsorption and Cr/MCM-41- impregnated XRD pattern |
| 表 1 Cr/MCM-41-ad和Cr/MCM-41-imp的XPS数据表 Table 1 XPS data of Cr/MCM-41-ad and Cr/MCM-41-imp |
图 6为Cr/MCM-41催化剂的Mapping图, 明亮区域为Cr分散.图(a)和图(b)显示Cr/MCM-41-imp上Cr相较于Cr/MCM-41-ad分散较差.
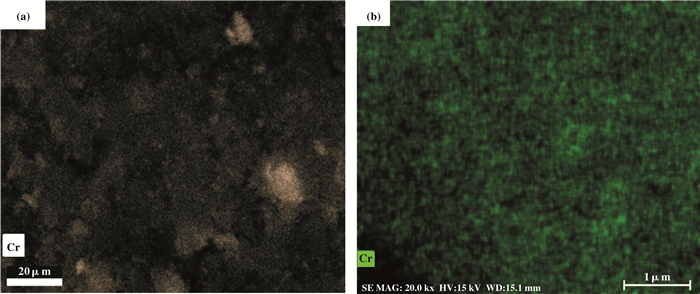
|
图 6 Cr/MCM-41-imp(a)与Cr/MCM-41-ad(b)催化剂的Mapping图 Figure 6 Mapping diagram of Cr/MCM-41-imp(a) and Cr/MCM-41-ad(b) catalysts |
图 7为Cr/MCM-41-ad和Cr/MCM-41-imp的XPS谱图, 与Cr 2p3/2相对应的结合能见表 1.所有催化剂在579和576 eV处均存在Cr6+和Cr3+的氧化状态[18-20]. Cr/MCM-41-ad和Cr/MCM-41-imp的Cr6+的结合能分别是577.5和576.46 eV, 由此得出的结论是, Cr/MCM-41-ad的Cr6+结合能高于Cr/MCM-41-imp, 说明Cr/MCM-41-ad上Cr与载体的相互作用较强[21].并且XPS图显示Cr/MCM-41-imp上的Cr(Ⅵ)比Cr/MCM-41-ad的多, 结合图 6说明在Cr/MCM-41-imp上易形成孤立态Cr(Ⅵ), 因此Cr/MCM-41-imp的活性低于Cr/MCM-41-ad.

|
图 7 Cr/MCM-41-ad (a)和Cr/MCM-41-imp (b)的XPS图 Figure 7 Cr/MCM-41-adsorption (a) and Cr/MCM-41-impregnated (b) XPS diagram |
通过Cr/MCM-41-ad和Cr/MCM-41-imp的UV-Vis吸收光谱发现在MCM-41表面上存在不同种类的氧化铬物种.图 8(a)结果所示, 在270和370 nm附近出现两个吸收峰, 为O→Cr(Ⅵ)的电荷转移[17, 22-24].而八面体Cr(Ⅲ)由于4A2g→4T1g, 4A2g→4T2g和4A2g→4T2g跃迁在370, 460和600 nm显示出3条谱带[23].在460 nm处的峰归属于Cr(Ⅵ)跃迁1T1←1A1(1t1→2e), 该带与四面体对称的Cr(Ⅵ)基团的扭曲密切相关[26].在600 nm处的谱带对应于α-Cr2O3的T2 g←A2 g跃迁, 结果表明样品中存在体相Cr2O3 [12, 17, 27].得到的UV-Vis结果与XRD结果一致.
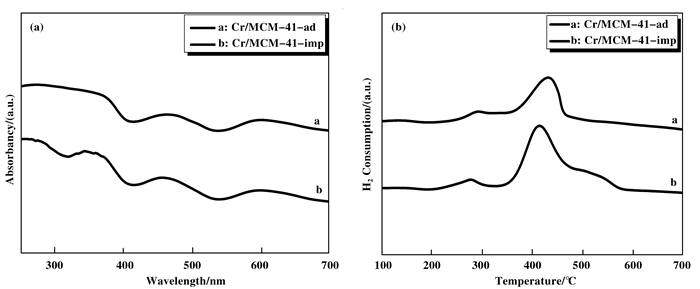
|
图 8 Cr/MCM-41-ad和Cr/MCM-41-imp的(a):UV图和(b):H2-TPR图 Figure 8 Cr/MCM-41-adsorption and Cr/MCM-41-impregnated (a):UV and(b):H2-TPR diagram |
图 8(b)为Cr/MCM-41-ad和Cr/MCM-41-imp的H2-TPR曲线. 250到280 ℃处出现的还原峰是由于分散在α-Cr2O3上的Cr(Ⅵ)的还原引起的[12], 这与所提到的XRD和UV-Vis的结果一致.在350~500 ℃出现的还原峰为Cr(Ⅵ)还原为Cr(Ⅲ)的还原峰[29-30]. TPR图显示, Cr/MCM-41-ad的还原峰位置相较于Cr/MCM-41-imp的后移, 是由于Cr/MCM-41-ad上的Cr与载体的相互作用较强[11], Cr在载体上分散好, 进而催化活性高[31].这与Mapping和XPS所得到的结果相一致.从图 8中可以看出, Cr/MCM-41-imp要比Cr/MCM-41-ad多一个525 ℃的肩峰, 在525 ℃出现的肩峰为孤立态的Cr(Ⅵ), 其比高分散Cr(Ⅵ)难还原, 是一种非活性态的Cr(Ⅵ)[29].其结果导致Cr/MCM-41-imp的活性低于Cr/MCM-41-ad.
3 结论 3.1合适的反应条件为温度630 ℃、空速4500 mL·h-1·g-1、压力0.1 MPa.合适的反应条件下丙烷转化率可以达到63%左右, 丙烯选择性为85%左右.
3.2通过对两种不同制备方法制得的催化剂进行活性比较实验, 可以得出, 由连续吸附法制得的Cr/MCM-41-ad的催化性能明显优于由传统浸渍法制得的Cr/MCM-41-imp.
3.3Cr/MCM-41-ad的催化性能优于Cr/MCM-41-imp.通过相关表征结果, 发现Cr/MCM-41-ad上的α-Cr2O3晶体粒径较小且分散均匀, 而Cr/MCM-41-imp催化剂存在非活性孤立态Cr(Ⅵ), 较难还原, 影响其反应活性.所以Cr/MCM-41-ad的活性好于Cr/MCM-41-imp.
| [1] |
a. BuTingting (卜婷婷), Li Qiuying (李秋颖), Ruan Wenjia (苟文甲), et al. Research progress of propane dehydrogenation Cr series catalysts (丙烷脱氢Cr系催化剂的研究进展)[J]. Petro Proce Chem Indus (C1 Chemis Chem Indus)(石油炼制与化工(C1化学与化工)), 2017, 48 (11): 103-110. b. Zhang Qiao(张巧), Zhang Ke-ting(张客厅), Wang Chen-guang(王晨光), et al. The performance of propane dehydrogenation over PtSn metal loaded on calcined Mg-Al layered double hydrotalcite (负载PtSn金属助剂的镁铝水滑石上的丙烷脱氢反应研究)[J]. J Mol Catal(China)(分子催化), 2018, 32 (4): 359-369. |
| [2] | Haifeng Xiong, Sen Lin, Joris Goetze, et al. Thermally stable and regenerable platinum-tin clusters for propane dehydrogenation prepared by atom trapping on ceria[J]. Angew Chemie-Inter Edit, 2017, 56: 8986–8991. DOI:10.1002/anie.201701115 |
| [3] | Xu Xin-pei(许鑫培), Wang De-long(王德龙), Yao Yue(姚月), et al. Research progress on chrome dehydrogenation of propane to propylene catalysts(丙烷脱氢制丙烯铬系催化剂研究进展)[J]. Nat Gas Chem Indus(天然气化工), 2017, 42(5): 107–113. DOI:10.3969/j.issn.1001-9219.2017.05.021 |
| [4] | Guo Xiao-hong(郭晓红), Li Ya-nan(李亚男), Zhou Guang-dong(周广栋), et al. Study on dehydrogenation of ethane to ethylene over CO2 oxide over supported Co-Cr oxide catalyst(负载型Co-Cr氧化物催化剂上CO2氧化乙烷脱氢制乙烯反应的研究)[J]. J Mol Catal(China)(分子催化), 2005, 19(6): 457–461. DOI:10.3969/j.issn.1001-3555.2005.06.009 |
| [5] | Shao Huai-qi(邵怀启), Zhong Shun-he(钟顺和). Study on dehydrogenation of propane in CO2 with MoO3-V2O5/SiO2 catalyst(CO2氧化丙烷脱氢MoO3-V2O5/SiO2催化剂研究)[J]. J Mol Catal(China)(分子催化), 2004, 18(2): 97–92. |
| [6] | Huang Yan(黄彦), Wang Guo-jia(王国甲), Yu Jian-feng(于剑锋), et al. Oxygen-free dehydrogenation and oxidative dehydrogenation of isobutane over Cr2 (MoO4)3, Fe2 (MoO4)3 catalyst(异丁烷在Cr2(MoO4)3、Fe2(MoO4)3催化剂上的无氧脱氢与氧化脱氢)[J]. J Mol Catal(China)(分子催化), 1997, 11(3): 221–225. |
| [7] |
a. Katsuomi Takehira, Yoshihiko Ohishi, Tetsuya Shishido, et al. Behavior of active sites on Cr-MCM-41 catalysts during the dehydrogenation of propane with CO2[J]. J Catal, 2004, 224: 404-416. b. Yi Ru(意如), Bai Sagala(萨嘎拉), Bao Zhaorigetu(照日格图). Preparation of Pd/MCM-41 and its photocatalytic performance for benzene hydroxylation(Pd/MCM-41催化剂的制备及其光催化苯羟基化的研究)[J]. J Mol Catal(China)(分子催化), 2016, 30 (6): 583-593. c. Wang Gai(王改), Yang Dong-hua(杨冬花), Bo Qiong(薄琼), et al. Synthesis and characterization of a multichanne porous MCM-41/Y composite zeolites(具有多级孔MCM-41/Y复合分子筛的合成及表征)[J]. J Mol Catal(China)(分子催化), 2018, 32 (4): 325-333. |
| [8] | Mentasty L R, Gorriz O F, Cadus L E. Chromium oxide supported on different Al2O3 supports:Catalytic propane dehydrogenation[J]. Indus & Engineer Chem Res, 1999, 38: 396–404. |
| [9] | Shee D, Sayari A. Light alkane dehydrogenation over mesoporous Cr2O3/Al2O3, catalysts[J]. Appl Catal A:Gener, 2010, 389(1): 155–164. |
| [10] | Michorczyk P, Ogonowski J, Zeńczak K. Activity of chromium oxide deposited on different silica supports in the dehydrogenation of propane with CO2-A comparative study[J]. J Mol Catal Chem, 2011, 349(1): 1–12. |
| [11] | Talati A, Haghighi M, Rahmani F. Impregnation vs. coprecipitation dispersion of Cr over TiO2 and ZrO2 used as active and stable nanocatalysts in oxidative dehydrogenation of ethane to ethylene by carbon dioxide[J]. Rsc Advan, 2016, 6(50): 44195–44204. DOI:10.1039/C6RA05049B |
| [12] | Fan Zhang, Runxia Wu, Yinghong Yue, et al. Chromium oxide supported on ZSM-5 as a novel efficient catalyst for dehydrogenation of propane with CO2[J]. Micro Mes Mater, 2011, 145: 194–199. DOI:10.1016/j.micromeso.2011.05.021 |
| [13] | Piotr Michorczyk, Jan Ogonowski, Piotr Kustrowski, et al. Chromium oxide supported on MCM-41 as a highly active and selective catalyst for dehydrogenation of propane with CO2[J]. Appl Catal A:Gener, 2008, 349: 62–69. DOI:10.1016/j.apcata.2008.07.008 |
| [14] | Saliha Kilicarslan, Meltem Dogan, Timur Dogu. Cr incorporated MCM-41 type catalysts for isobutane dehydrogenation and deactivation mechanism[J]. Indus & Engineer Chem Res, 2013, 52: 3674–3682. |
| [15] | Adolfovich L A, Nailevna M A, Eduardovich B G, et al. Modification by SiO2 of alumina support for light alkane dehydrogenation catalysts[J]. Catal, 2016, 6: 1–6. |
| [16] | Lu Jichang, Hao Husheng, Zhang Liming, et al. The investigation of the role of basic lanthanum (La)species on the improvement of catalytic activity and stability of HZSM-5 material for eliminating methanethiol (CH3SH)[J]. Appl Catal B:Environ, 2018, 237: 185–197. DOI:10.1016/j.apcatb.2018.05.063 |
| [17] | He Dedong, Zhang Liming, Zhao Yutong, et al. Recycling spent Cr adsorbents as catalyst for eliminating methylmercaptan[J]. Environ Sci & Technol, 2018, 52: 3669–3675. |
| [18] | Shi Xuejun, Ji Shengfu, Wang Kai, et al. Oxidative dehydrogenation of ethane with CO2 over novel Cr/SBA-15/Al2O3/FeCrAl monolithic catalysts[J]. Ener Fuels, 2008, 22(6): 3631–3638. DOI:10.1021/ef800567v |
| [19] | Kilicarslan S, Dogan M, Dogu T. Cr incorporated MCM-41 type catalysts for isobutane dehydrogenation and deactivation mechanism[J]. Indus & Engineer Chem Res, 2013, 52(10): 3674–3682. |
| [20] | Hoang D L, Dittmar A, Radnik J, et al. Redox beha-viour of La-Cr compounds formed in CrOx/La2O3, mixed oxides and CrOx/La2O3/ZrO2, catalysts[J]. Appl Catal A:Gener, 2003, 239(1): 95–110. |
| [21] | Merryfield R, Mcdaniel M, Parks G. An XPS study of the phillips Cr/silica polymerization catalyst[J]. J Catal, 1982, 77(2): 348–359. DOI:10.1016/0021-9517(82)90178-6 |
| [22] | Dedong He, Jie Yu, Yi Mei, et al. The effects of Cr addition in HZSM-5 on its structure, physicochemical and catalytic properties for methyl mercaptan abatement[J]. Catal Commun, 2018, 112: 31–34. DOI:10.1016/j.catcom.2018.04.013 |
| [23] | Rossi S De, Casaletto M, Ferraris G, et al. Chromia/zirconia catalysts with Cr content exceeding the monolayer. A comparison with chromia/alumina and chromia/silica for isobutane dehydrogenation[J]. Appl Catal A:Gener, 1998, 167: 257–270. DOI:10.1016/S0926-860X(97)00315-3 |
| [24] | Zhao X, Wang X. Synthesis, characterization and catalytic application of Cr-SBA-15 mesoporous molecular sieves[J]. J Mol Catal A:Chem, 2007, 261: 225–231. DOI:10.1016/j.molcata.2006.08.008 |
| [25] | Weckhuysen B M, Verberckmoes A A, Debaere J, et al. In situ UV-Vis diffuse reflectance spectroscopy-on line activity measurements of supported chromium oxide catalysts:relating isobutane dehydrogenation activity with Cr-speciation via experimental design[J]. J Mol Catal A:Chem, 2000, 151: 115–131. DOI:10.1016/S1381-1169(99)00259-9 |
| [26] | Santamaría-González J, Mérida-Robles J, Alcántara-Rodríguez M, et al. Catalytic behaviour of chromium supported mesoporous MCM-41 silica in the oxidative dehydrogenation of propane[J]. Catal Lett, 2000, 64: 209–214. DOI:10.1023/A:1019019927560 |
| [27] | Cavani F, Koutyrev M, Trifirò F, et al. Chemical and physical characterization of alumina-supported chromia-based catalysts and their activity in dehydrogenation of isobutane[J]. J Catal, 1996, 158: 236–250. DOI:10.1006/jcat.1996.0023 |
| [28] | Lei Zhang, Yanhui Zhao, Hongxing Dai, et al. A comparative investigation on the properties of Cr-SBA-15 and CrOx/SBA-15[J]. Catal Today, 2008, 131: 42–54. DOI:10.1016/j.cattod.2007.10.017 |
| [29] | Jayeon Baek, Hyeong Jin Yun, Danim Yun, et al. Pre-paration of highly dispersed chromium oxide catalysts supported on mesoporous silica for the oxidative dehydrogenation of propane using CO2:Insight into the nature of catalytically active chromium sites[J]. ACS Catal, 2012, 2: 1893–1903. DOI:10.1021/cs300198u |
| [30] | Santamaría-González J, Mérida-Robles J, Alcántara-Rodríguez M, et al. Catalytic behaviour of chromium supported mesoporous MCM-41 silica in the oxidative dehydrogenation of propane[J]. Catal Lett, 2000, 64(2/4): 209–214. DOI:10.1023/A:1019019927560 |
| [31] | Fang D, Zhao J, Liu S, et al. Relationship between Cr-Al Interaction and the performance of Cr-AlO catalysts for isobutane dehydrogenation[J]. Mod Res Catal, 2015, 4(2): 50–58. DOI:10.4236/mrc.2015.42007 |
 2018, Vol. 32
2018, Vol. 32


