The origin of the elements in the universe is one of the most basic scientific questions. According to our current knowledge, the universe originated from the Big Bang, which created some light elements, such as hydrogen, helium, and a very small amount of lithium within very short time of this incredible process[1-3]. After then, more than 80 elements are generated via a series of complicated nuclear reactions under very harsh conditions[3]. Radioactive decay or natural nuclear reaction might also lead to formation of some elements or their isotopes, such as U to Pd[4-6]. It is known that the 40Ar and small amount of radio nuclides 14C are formed by constantly bombing under the high energy cosmic rays in the Earth's atmosphere[4-6]. Of course, we can produce some of element else from target element by high energy neutron and proton bombing typically in accelerators or Tokamak[7-10]. However, there are some mysterious questions related to the element abundance in our universe, for example, current understanding can not account for measured excess helium element abundance in our universe[11]. Scientists found that there were excess ratios of 3He and 4He insubmarine hydrothermal water[12]. The investigation of energy balance of Jupiter also seems indicate the possibility of low energy fusion reaction there[13].
It is well known that one element or its isotope can be converted into another element or isotope by nuclear transmutation[14]. In 1919, Rutherford reported the first artificial transmutation from nitrogen into oxygen by alpha particle bombing[15]. In 1932, John Cockcroft and Ernest Walton fulfilled an artificial nuclear reaction by 7Li bombing with accelerated protons to split the Li nucleus into two alpha particles[6]. In 1938, Otto Hahn et al. discovered artificial heavy element transmutation, 197Au+n→198Au, finally→204Ti and204Pb[9]. In fact, scientists found that transmutation can be induced in "mild" reactive systems, like in metabolism processes of vegetal and animal organisms[16], in which some elemental atoms are transmuted into the different ones while discharging energy slowly. Kervran et al. investigated potassium and calcium content variation during the growth of 840 seeds and 403 sprouts in biological systems, and found potassium may transmute into calcium during the process of seeds growing, 39K + 1p = 40Ca +E[17]. Some other elemental transmutations were also reported, such as sodium to magnesium (1123Na + n → 1124Na → 1224Mg + e- + ve*) and manganese to iron(2555Mn + n → 2556Mn → 2656Fe + e- + ve*)[17]. Those investigations imply that the elemental transmutation is essential to maintain a balance of certain elements in the biological bodies. Later on, much more reports of element transmutation by chemical or physical was appeared, for instance, the so-called cold fusion experiments which was done by Fleischmann and Pons, in which they found that trace amount of He could be produced by electrochemical method under normal conditions while excess heat was generated[18].
We previously reported that deuterium and helium were generated from protons by low energy nuclear reaction under light irradiation[19-23] and the transmutation of potassium element to calcium during photochemical reaction of hydrogen evolution (HER) in the presence of hydride (negative hydrogen ions—H-, which is offered by negative hydrogen compounds such as NaBH4, LiBH4 and NH3BH3[22]). Potassium was chosen as the target transmutation element because of the essential roles of potassium and calcium in biological bodies.
In this work, we found interesting results about the concentration variation of Ba isotopes in the presence of hydride and CsCl. Experimental results showed that the significant concentration and ratio variation of 130Ba, 132Ba, 134Ba, 135Ba, 136Ba, 137Ba and 138Ba isotopes after very short time reaction.Especially, the changes of 130Ba and 132Ba concentration are remarkable compared with other isotopes. These results are extremely meaningful for understanding the elements evolution on the Earth, as well as providing new way to looking into the low energy nuclear reaction.
1 Experimental detailsAll chemicals were purchased and used without further purification, NaBH4 (Shanghai Guangming Chemical Reagent Co., Ltd, AR, ≥ 97 %), CsCl (Kemiao Chemical Co., Ltd., AR, ≥ 99.5%), RuCl3 (Shanghai Guangming Chemical Reagent Co., Ltd, AR, ≥ 37.3%), and BaCl2 (Xilong Chemical Works, AR, ≥ 99.5%). Ultrapure water: 18.2 MΩ·cm-1 at 25 ℃ (Milli-Q water, Millipore Mili-Q reference ultrapure water purification system, USA) was used in this study. The polypropylene (PP) volumetric flask was used in this work since it is chemical stableboth in the acidic and alkaline environment. The involvment of other elements can be eliminated after very careful checking.
Prior to the reaction, all PP volumetric flasks (100 mL) were recalibrated and washed several times with high pure Mill-Q water, and then they were dried at room temperature. The typical isotope trasmutation (Cs-Ba) experiment was done as follows. 50.0 mL of ultrapure water, 1.0 mL of CsCl solution (CCs=200 mg· kg-1) and 2.0 mL RuCl3 solution (20 mg·mL-1) were added in a 100 mL PP volumetric flask. After 5 min ultrasound treatment, different content NaBH4 was added in the mixture solution, and the reaction was operated for days until no bubbles were generated under the room temperature. After that, the mixed solution was volumed to 100 mL with Mill-Q high purity water. For the above reaction system, it was labeled as NaBH4+CsCl+RuCl3. The experimental results are shown in Fig. 1.

|
Fig.1 (a) Ba isotope concentration varation in NaBH4+CsCl+RuCl3 mixture with different NaBH4 content 236.4 and 236.4×4 mg; (b) the corresponding isotope ratio |
The element and isotope concentration and ratio variation before and after reaction were measured by inductive coupled plasma mass spectrometry (ICP-MS, iCAP Q, Thermo, Waltham, USA) technique. The apparatus limits of detection for Ba and Cs isotope are 0.40 and 0.05 ppt respectively. The samples for ICP-MS measurement were not further diluted prior to analysis. The every sample was detected at least three times and the average value was presented.
2 Results and discussionICP-MS measurement results are shown in Fig. 1a and Table 1. The reaction mixture contains 1 mL CsCl (CCs=200 mg·kg-1) and 2.0 mL RuCl3(20 mg·mL-1) solution in 100 mL PP volumetric flask. By changing the amount of NaBH4 that are 236.4 and 236.4 ×4 mg, respectively, we found that the Ba isotope concentration changed with the increase of negative hydrogen compound loading (NaBH4). It should be noted that there is no additional barium source in these initial reaction mixtures.Meanwhile, the Ba isotope ratio between 130Ba, 132Ba, 134Ba, 135Ba, 136Ba, 137Ba and 138Ba also changed after the raection, as shown in Fig. 1b. In particular, the amount of isotope 130Ba and 132Ba decrease remarkably with the increase of negative hydrogen compound loading.
| Table 1 Ba isotope concentration varation in the mixture of NaBH4+CsCl+RuCl3 with different NaBH4 loading |
To avoid misunderstanding of the experimental results, we double checked the initial Ba and Cs isotopeconcentration in NaBH4, RuCl3, CsCl and BaCl2 solu- tions by ICP-MS. As results shown in Fig. 2a, the Ba isotope concentrations of 132Ba, 134Ba, 135Ba, 136Ba, 137Ba and 138Ba in NaBH4 and CsCl solution are lower than the instrument's limit of detection. Only a trace amount 130Ba was found in NaBH4(1.41×10-3 mg·kg-1) and CsCl(2.03×10-3 mg·kg-1) solution respectively. The concentrations of the Ba isotopes in RuCl3 aqueous solution are also very low, as shown in Table 2. This means that the changing of Ba isotope concentration can only be ascribed to elemental transmutation reaction in the NaBH4+CsCl+RuCl3 mixture. The distribution of isotope ratio in BaCl2 aqueous solution(CBa=132.0×10-3 mg·kg-1)was also detected, and detected 130Ba concentration in BaCl2 is significantly lower than other isotope concentrations.

|
Fig.2 The Ba isotope concentration in used regents (a) Ba isotope concentrations in the NaBH4, RuCl3, CsCl and BaCl2 solution respectively; (b) the corresponding isotope ratio in the NaBH4, RuCl3, CsCl and BaCl2 solution respectively |
| Table 2 Ba isotope concentrations in NaBH4, RuCl3, CsCl3 and BaCl2 solution |
Fig. 3a shows the Ba isotope concentrations in NaBH4+CsCl+RuCl3 and NaBH4+CsCl mixtures with different CsCl concentrations. We found that the Ba isotope concentrations, such as 130Ba and 132Ba, are obvious different in two reaction mixtures(with or without RuCl3) as shown in Table 3. The results indicates that the Ru has a catalytic effect on the Ba isotope concentration change. Moreover, increasing the initial CsCl concentration, the contents of Ba isotopes also change significantly in the NaBH4+CsCl+RuCl3 or NaBH4+CsCl mixture. In short, introduction of catalyst Ru and Cs are decisive for 130Ba and 132Ba isotope conversion. By comparison the Ba isotope ratio in Fig. 3b, it is confirmed that the isotope concentration distribution of Ba is closely related to Ru and Cs addition.
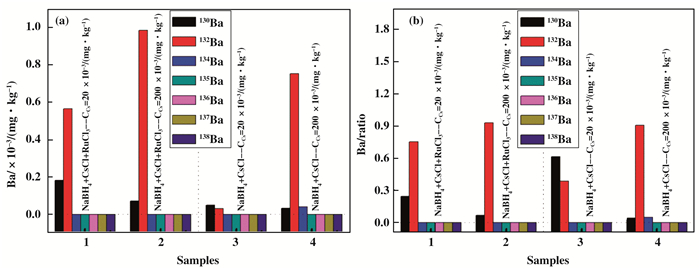
|
Fig.3 (a) Ba isotope concentrations in NaBH4+CsCl+RuCl3 and NaBH4+CsCl mixtures with different Cs content(CCS=20×10-3mg·kg-1)(1#, 2#) and (CCS=200×10-3 mg·kg-1) (3#, 4#); (b) the corresponding isotope ratio in their respective mixtures |
| Table 3 Ba isotope concentrations in NaBH4+CsCl+RuCl3 and NaBH4+CsCl mixture |
Does the variation of Ba isotope concentration and ratio result from interaction of Ba itself and hydride? We conducted a reaction of hydride with Ba in the absence of Cs. In NaBH4+BaCl2+RuCl3 mixture, the Ba isotope concentration almost maitained similar under different hydride loading, according to results shown in Fig. 4 and Table 4. That confirmed that the concentration variation was not due to of the reaction between Ba isotopes with negative hydrogen (NaBH4), instead, from reaction between Cs and hydride, i.e., mCs + H·-→nBa.

|
Fig.4 Ba isotope concentrations in NaBH4+BaCl2+ RuCl3 mixtures detected by ICP-MS incyclic experiments at 25 ℃, (Ⅰ) 1 cycle, [NaBH4]: 236.4 mg; (Ⅱ) addition of another portion of NaBH4 (236.4 mg). Initial reactants: mRuCl3=40 mg and CBa=132×10-3 mg·kg-1 |
| Table 4 Ba isotope concentrations in NaBH4+BaCl2+RuCl3 mixtures detected by ICP-MS, (Ⅰ) 1 cycle, [NaBH4]: 236.4 mg; (Ⅱ) addition of fresh NaBH4 (236.4 mg) |
This work reported evidences of Cs to Ba transmutation in the presence of hydride compounds. Experiments identified that the concentration and isotope ratio were changed after reaction of Cs salt with hydride at room temperature. In addition, we confirm that these phenomena are closely related to Ru catalyst role. Those results imply that some of barium isotope in nature might originate from an unknown low-energy nuclear reaction between Cs and hydride under very mild conditions.
AcknowledgementsThis work is supported by the National Natural Science Foundation of China (Grant Nos. 21673262 and 21433007), respectively.
| [1] |
Burbidge E M, Burbidge G R, Fowler W A, et al. Synthesis of the elements in stars[J]. Rev Mod Phys, 1957, 29(4): 547.
|
| [2] |
Fowler W A. Experimental and theoretical nuclear astrophysics: The quest for the origin of the elements[J]. Mod Phys, 1984, 56(2): 149.
|
| [3] |
Woosley S E, Heger A, Weaver T A. The evolution and explosion of massive stars[J]. Mod Phys, 2002, 74(4): 1015.
|
| [4] |
Badash L. Radium, radioactivity, and the popularity of scientific discovery[J]. Proc Am Philos Soc, 1978, 122(3): 145–154.
|
| [5] |
Howorth M. Pioneer research on the atom: Rutherford and soddy in a glorious chapter of science; the life story of Frederick Soddy[M]. New World Publications, 1958.
|
| [6] |
Trenn T J. The self-splitting atom: the history of the Rutherford-Soddy collaboration[M]. London: Taylor & Francis, 1977, 42 : 58-60; 111-117.
|
| [7] |
Cockcroft J, Walton E. Artificial Production of Fast Protons (Reprinted from Nature, February 13, 1932)[J]. Nature, 1969, 224: 463.
|
| [8] |
Henderson M. The disintegration of lithium by protons of high energy[J]. Phys Rev, 1933, 43(2): 98–102.
|
| [9] |
Paneth F A, Günyher P L. Chemical detection of artificial transmutation of elements[J]. Nature, 1933, 131: 652–653.
|
| [10] |
Bush R P. Recovery of platinum group metals from high level radio active waste[J]. Platinum Metals Rev, 1991, 35: 202–208.
|
| [11] |
Yoshida N, Oh S P, Kitayama T, et al. Early cosmological H2/3He regions and their impact on second-generation star formation[J]. Astrophys J, 2007, 663: 687–707.
|
| [12] |
Craig H, Clarke W B, Beg M A. Excess 3He in deep water on the east pacific rise[J]. Earth Planet Sci Lett, 1975, 26: 125–132.
|
| [13] |
Hanel R A, Conrath B J, Herath L W, et al. Albedo, internal heat, and energy balance of jupiter: preliminary results of the voyager infrared investigation[J]. J Geophys Res, 1981, 86(A10): 8705–8712.
|
| [14] |
Schaeffer O A, Zähringer J. Solar flare helium in satellite materials[J]. Phys Rev Lett, 1962, 8: 389.
|
| [15] |
Badash L. Radium, radioactivity and the popularity of scientific discovery[J]. Proc Am Philos Soc, 1978, 122: 145–154.
|
| [16] |
Kervran C L. Biological transmutations[M]. (revised and edited by H. Rosenauer and E. Rosenauer, Crosby Lockwood, London 1972, reprinted by Beekman, New York, 1980, reprinted 1998).
|
| [17] |
Biberian J P. Biological transmutations[J]. Curr Sci, 2015, 108: 633–635.
|
| [18] |
Fleischmann M, Pons S. Electrochemically induced nuclear fusion of deuterium[J]. J Electroanal Chem Interf Electrochem, 1989, 261: 301–308.
|
| [19] |
Lu Gong-xuan(吕功煊), Tian Bin(田彬). Formation of deuterium and helium during photocatalytic hydrogen generation from water catalyzed by Pt-graphene sensitized with Br-dye under visible light irradiation(溴染料敏化担载Pt石墨烯催化可见光制氢、氘和氦)[J]. J Mol Catal (China)(分子催化), 2017, 31(2): 101–104.
|
| [20] |
Lu Gong-xuan(吕功煊), Zhen Wen-long(甄文龙). Formation of deuterium and helium during photocatalytic hydrogen generation from water catalyzed by Pt-Graphene sensitized with Br-dye under visible light irradiation(半导体CdS悬浮体系中可见光催化产氢同时生成氦-3和氦-4)[J]. J Mol Catal (China)(分子催化), 2017, 31(4): 299–304.
|
| [21] |
Lu Gong-xuan(吕功煊), Zhang Wen-yan(张文妍). Photocatalytic hydrogen evolutionand induced transmutation of potassium to calcium via low-energy nuclear reaction (LENR) driven by visible light(可见光驱动的光催化产氢同时诱导低能核反应嬗变钾为钙)[J]. J Mol Catal (China)(分子催化), 2017, 31(5): 401–410.
|
| [22] |
Lu Gong-xuan(吕功煊), Zhang Xu-qiang(张旭强). The detection of K-Ca transmutation in the mixture of K and hydride chemicals(关于在钾与负氢混合物中钾钙嬗变的检测)[J]. J Mol Catal (China)(分子催化), 2019, 33(1): 1–9.
|
 2019, Vol. 33
2019, Vol. 33

