丙烯是一种重要的石油化工原料, 主要生产途径有蒸汽裂解、煤或甲醇转化以及丙烷直接脱氢(Dehydrogenation of propane, DHP)等[1-2].近年来, 天然气和页岩气开采量加大, 利用其副产物丙烷直接脱氢工艺生产丙烯比例逐年上升[3].在基础研究层面, 因丙烷氧化脱氢(Oxidative dehydrogenation of propane, ODHP)过程较DHP过程在热力学上更为有利, 而受到研究者普遍重视, 但却受制于高温条件下丙烯易深度氧化、裂解等瓶颈[3-4].应对这一挑战, 一些研究者探索发现纳米碳材料[5]、六方BN等[6]非金属催化剂具有优异的丙烯选择性; 同时也有研究者设法调控传统过渡金属氧化物催化剂组成、形貌和结构等物性, 从而改善其ODHP催化性能[3, 7-12].其中, 以类水滑石(即层状双金属氢氧化物, Layered double hydroxide, LDH)为前驱体焙烧所得高分散多组分纳米混合金属氧化物(Mixed metal oxide, MMO), 如Mg-Al-V[7]、Ni-Al[8]、Ni-Al-V[9]、Mg-Co-Al[10]、Co-Al[11]、Zn-Al-V[12] MMO等, 是一类具有开发潜质的ODHP催化剂.这主要是因为此类材料具有比表面积大、金属元素分散度高及酸碱度可调等优良特性[13].如Smoláková L等[8]考察了Ni含量对催化剂ODHP催化性能的影响, 认为增加Ni含量, 活性物种分散性降低, 丙烯选择性随反应温度升高明显下降. Huang M X等[11]研究2CoAl MMO的ODHP催化性能发现层间阴离子(SO42-, PO43-)存在有利于提高CoOx分散性, 从而提高其丙烯选择性. Mitran G等[10]将Co掺杂于3Mg-Al LDH中高温焙烧获得3(MgCox)Al MMO, 认为Co含量较低时分散性较好的四面体配位Co物种是催化活性中心, 可获得较高的丙烯选择性, Co含量较高时形成Co3O4尖晶石相利于COx生成, 导致催化剂丙烯选择性降低.
我们通过文献调研发现, 一方面, Ni-Co体系催化性能研究已有报道, 如Sun Y F等[14]用一锅法制备了10Ni10Co-介孔Al2O3催化剂, 发现其ODHP催化性能(450 ℃, 丙烯收率10.3%)优于浸渍法所得催化剂(450 ℃, 丙烯收率2.4%); Cai T等[15]研究表明, 由简单共沉淀法所制备的Ni-Co-O固溶体纳米晶在低温(170 ℃)丙烷燃烧反应中的比速率是纯Co3O4的2.5倍.另一方面, 以LDH为前驱体的Ni基MMO催化剂仍存在高温丙烯选择性明显降低的问题, 但利用调变组成改善其ODHP催化性能的研究未见报道.我们长期致力于Ni基ODHP催化剂的研究[16], 采用恒定pH共沉淀法制备不同Ni/Co摩尔比的3(NixCo1-x)-Al LDH前驱体, 经600 ℃焙烧得3(NixCo1-x)Al MMO催化剂, 考察Ni/Co摩尔比对其ODHP催化性能的影响, 并通过X射线光电子能谱(XPS)、H2程序升温还原(H2-TPR)和原位电导测试等探究其原因.
1 实验部分 1.1 原料与试剂实验所用试剂(分析纯): NiCl2·6H2O, CoCl2· 6H2O, AlCl3·6H2O(阿拉丁试剂有限公司); NaOH(天津市北联精细化学品开发有限公司); 无水Na2CO3(天津市盛奥化学试剂有限公司); 无水C2H5OH(天津永晟精细化工有限公司).
1.2 样品制备 1.2.1 3(NixCo1-x)Al LDH前驱体制备采用恒定pH共沉淀法[13a, 17]制备系列3(NixCo1-x)Al LDH前驱体(摩尔比M2+/Al3+=3:1).以3(Ni0.95Co0.05)Al LDH为例.取NiCl2·6H2O 42.75 mmol, CoCl2·6H2O 2.25 mmol和AlCl3·6H2O 15 mmol加一定量二次水溶解后于250 mL容量瓶定容得溶液1.取Na2CO3 50 mmol和NaOH 200 mmol加一定量二次水至完全溶解得溶液2.取Na2CO3 1 mmol加100 mL二次水至完全溶解得溶液3.将溶液1逐滴加入溶液3中, 用溶液2调节混合液pH为10±0.2, 得浅绿色悬浊液, 室温静置陈化24 h, 离心, 水洗至上清液pH值至中性, 将固体于60 ℃干燥过夜.研磨备用, 标记为3(Ni0.95Co0.05)Al LDH.采用相同的制备方法, 通过调变Ni/Co比例, 制备一系列3(NixCo1-x)Al LDH前驱体(x=1, 0.95, 0.90, 0.75, 0.50, 0.25, 0).
1.2.2 3(NixCo1-x)Al MMO制备在马弗炉中将3(NixCo1-x)Al LDH样品于空气气氛600 ℃焙烧4 h, 升温速率2 ℃/min, 得3(NixCo1-x)Al MMO催化剂, 标记为3(NixCo1-x)Al MMO (x=1, 0.95, 0.90, 0.75, 0.50, 0.25, 0).
1.3 样品物性表征Ni, Co含量分析:电感耦合等离子体发射光谱仪(ICP-MS, 美国Thermoscientific iCAP RQ).表面织构分析:孔结构及比表面积分析仪(美国Micromeritics公司ASAP2020), 样品100 ℃真空脱气预处理5 h, 液氮77 K静态N2物理吸附-脱附, 8点Brunauer-Emmett-Teller (BET)模型计算得样品比表面积.样品晶相分析: X射线衍射仪(XRD, 日本理学Rigaku Ultima IV), Cu靶, Kα辐射源(λ=0.1540 nm), 扫描速度10 °/min, 扫描范围5°~80°, 管电压40 kV, 管电流40 mA.形貌分析:场发射透射电子显微镜(TEM, 美国FEI公司Tecnai G2 F20), 电压200 kV. H2-TPR:多功能化学吸附分析仪(美国Quantachrome公司, Chem BET TPR/TPD), 热导率检测器(TCD), 催化剂50 mg, 还原气10%H2/Ar混合气, 升温速率10 ℃·min-1.表面金属价态分析: X射线光电子能谱仪(XPS, 美国Thermo-Fisher公司的ESCALAB 250Xi), 单色化X射线源, Al阳极靶(1486.6 eV), 功率150 W, 结合能校准C 1s 284.8 eV.固体电导分析:采用自主搭建多功能电导分析仪(Fluke8840A)测定电阻值, 电导率σ (s·m-1) =L/(R·S) (R/Ω:电阻, L/m :样品片厚度, S/m2:样品横截面积)[16a]; 改变氧分压PO2测定电导率σ, 得dσ/dPO2>0, 表明3(NixCo1-x)Al MMO均为p型半导体氧化物[18]; 450 ℃, 通过循环切换不同气氛(N2+O2 → N2+O2+C3H8), 测定催化剂的原位电导率变化.
1.4 催化活性评价催化剂的ODHP催化性能测试在固定床石英管反应器中完成, 催化剂装量0.2 g, 常压, 反应温度350~550 ℃, C3H8/O2/N2体积比为2:2:16, 总流量20 mL·min-1, 空速(GHSV)6000 mL·g-1·h-1.采用岛津GC-2010 Plus气相色谱仪(ShinCarbon ST 80/100 mesh微填充柱, BID检测器)对反应物和产物进行分析.空白实验表明在测试温度范围内丙烷无转化.催化剂寿命实验条件: C3H8:O2:N2=2:2:16, T = 450 ℃, 连续在线测试12 h.
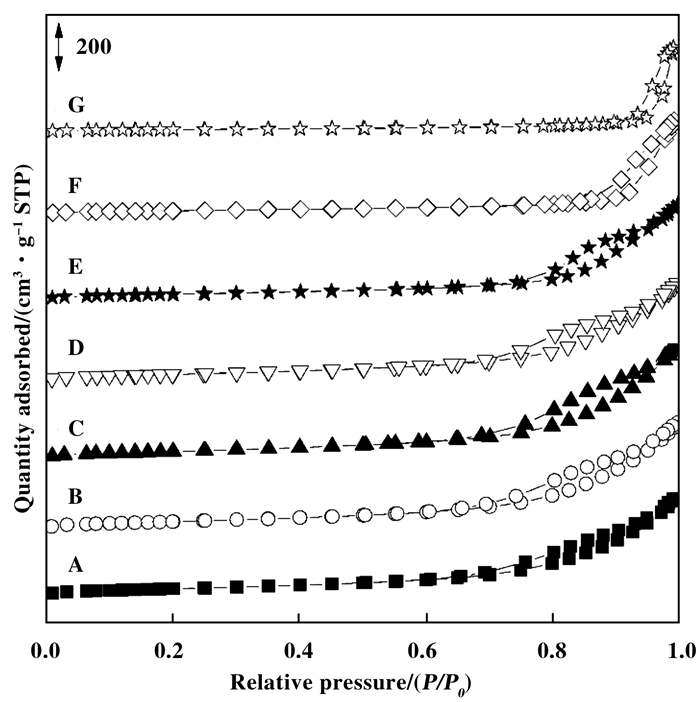
2 结果与讨论 2.1 样品物性表征结果 2.1.1 样品的组成和表面织构由表 1催化剂组成数据可见, Ni/Co摩尔比与理论值一致, 表明合成LDH前驱体过程Ni2+和Co2+共沉淀完全.图 1为催化剂的N2吸附-脱附等温曲线图.根据IUPAC分类[19], 所制备的3(NixCo1-x)Al MMO催化剂均呈现典型的层状粘土所具有的Ⅱ型吸附-脱附等温线, 并伴有H3型迴滞环, 无均匀孔径分布.随Co含量增加, 迴滞环闭合点明显向高比压区移动, 表明层片尺寸变大, 层片堆叠形成的狭缝也相应变宽.由表 1比表面积和孔容数据可见, Co含量较低(x≥0.90), 比表面积和孔容先略有增大, 但当Co含量增加(x≤0.90), 二者均明显减小.
| 表 1 催化剂的化学组成、比表面积及孔容 Table 1 Chemical composition, specific area and pore properties of 3(NixCo1-x)Al MMO catalysts |
|
图 1 3(NixCo1-x)Al MMO的N2吸附-脱附等温曲线 Fig.1 N2 adsorption-desorption isotherms of 3(NixCo1-x)Al MMO A~G: x=1, 0.95, 0.90, 0.75, 0.50, 0.25, 0 |
图 2为3(NixCo1-x)Al LDH前驱体(a)和3(NixCo1-x)Al MMO (b)的XRD谱图.在图 2 a中, 2θ为11.3°、22.8°、34.7°、39.1°、60.6°、61.9°处出现LDH的特征衍射峰, 分别对应(003)、(006)、(012)、(015)、(110)和(113)晶面(JCPDS 35-0965)[20], 且峰型宽化, 表明合成的3(NixCo1-x)Al LDH晶粒均较小; 随着Co掺杂量增大, 衍射峰强度顺序递减, 表明在该合成条件下Co(Ⅱ)可替代部分Ni(Ⅱ)形成Ni-Co-Al三元LDH, 但大量引入Co(Ⅱ) LDH结晶度降低.另外, 因Ni(Ⅱ)和Co(Ⅱ)离子半径非常接近(0.72 vs. 0.78 [15]), 故以上LDH衍射峰位随Co(Ⅱ)掺杂并未发生明显偏移. LDH前驱体经600 ℃焙烧后所得MMO催化剂仅呈现对应氧化物特征衍射峰, 这是由于焙烧温度相对较低, 不能形成Al2O3、NiAl2O4和CoAl2O4尖晶石等晶相[20-21].另外, 在LDH焙烧形成MMO过程中Al(Ⅲ)会少量进入所形成的氧化物晶相中, 形成类氧化物晶相, 但因引入Al(Ⅲ)量较少导致很难分辨其特征衍射峰变化[8, 11].由图 2 b可见, 随Co含量增加, 类NiO相特征衍射峰(JCPDS 75-0197) 37.3° (111)、43.4°(200)和63.0° (220)强度逐渐减弱, Co(Ⅱ)可能取代部分Ni(Ⅱ)进入类NiO晶相; 当x=0.75时开始出现类Co3O4相特征衍射峰(JCPDS 74-1656), 分别为19.0° (111)、31.3°(220)、36.9° (311)、44.9° (400)、59.5° (511)和65.4° (440).当x=0.50时, 则明显以类Co3O4相为主.当x=0.25时, 已经完全观测不到类NiO相, 即Ni(Ⅱ)可能替代部分Co(Ⅱ)进入类Co3O4晶相.

|
图 2 3(NixCo1-x)Al LDH (a)和3(NixCo1-x)Al MMO (b)的XRD谱图 Fig.2 XRD patterns of 3(NixCo1-x)Al LDH (a) and 3(NixCo1-x)Al MMO (b) A~G: x=1, 0.95, 0.90, 0.75, 0.50, 0.25, 0 |
图 3为部分3(NixCo1-x)Al MMO的TEM图像.由图可见, 当x < 0.50时(图 3A~C), Ni-Co-Al MMO形貌保持Ni-Al MMO特征, 为二维层片基底上的针状结构(可能是二维层板卷曲所致), 而当x ≥ 0.50时(图 3D、E), 其形貌明显出现Co-Al MMO特征, 为不规则较大层片结构, 且3CoAl MMO层片明显团聚.这与上述N2吸附-脱附以及XRD表征结果吻合.

|
图 3 3(NixCo1-x)Al MMO的TEM图像 Fig.3 TEM images of 3(NixCo1-x)Al MMO A~E: x=1, 0.90, 0.75, 0.50, 0 |
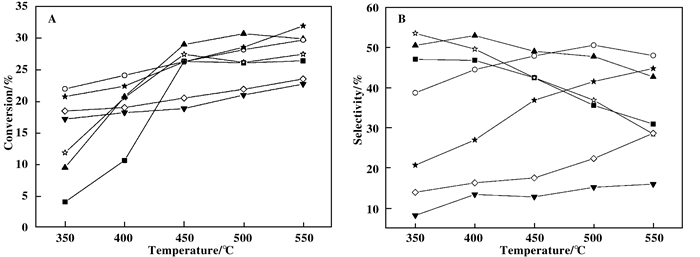
图 4给出3(NixCo1-x)Al MMO催化剂的ODHP催化性能测试结果.图 4 A和B分别为丙烷转化率和丙烯选择性随反应温度的变化关系.由图 4A可见, 在350~550 ℃测试范围内, 当Co掺杂量较小(x=0.95, 0.90)时, 3(NixCo1-x)Al MMO催化剂上丙烷转化率随温度变化规律与3NiAl MMO相同, 350~450 ℃间丙烷转化率迅速增大至25%以上, 450 ℃以上基本平稳; 但不同的是, 掺杂Co后低温丙烷转化率较掺杂前明显增大.当x=0.75时, 低温丙烷转化率达22%以上, 继续增大Co含量反而使低温丙烷转化率降低, 但仍高于3CoAl MMO; 同时当x≥0.75时, 由于低温丙烷转化率增大, 使丙烷转化率随反应温度升高而增大的变化率明显变小, 与3CoAl MMO上丙烷转化率随温度变化规律逐渐趋于一致.总体而言, 少量Co掺杂(x≥0.50)有利于提升低温丙烷转化率, 并可保持相对较高的高温丙烷转化率.由图 4B可见, Co掺杂对丙烯选择性的影响规律与其对丙烷转化率的影响规律类似. 3NiAl MMO上丙烯初始选择性(50%)明显高于3CoAl MMO(< 10%), 但前者随温度升高明显降低, 而后者则仅略有升高.掺杂少量Co(x=0.95)时, 低温丙烯选择性有所提升, 但高温时与3NiAl MMO趋于一致.有意思的是, 当x=0.90时, 即Ni/Co摩尔比=9:1时, 丙烯初始选择性(50%)高于3NiAl MMO且随反应温度升高降低不明显, 550 ℃时仍可保持在42%以上.继续增加Co含量(x=0.75, 0.50, 0.25), 丙烯初始选择性低于3NiAl MMO且依次减小, 但随反应温度升高, 丙烯选择性提高.综合对比, 500 ℃时3(Ni0.90Co0.10)Al MMO上ODHP催化性能最佳, 丙烷转化率31%, 丙烯选择性48%, 明显优于3NiAl MMO和3CoAl MMO在相同条件下的催化性能.文献研究表明, Ni基催化剂因表面存在高活性NiO相而普遍使高温丙烯选择性迅速下降[8]; Co基催化剂中Co3O4相存在利于生成COx, 但丙烯选择性随温度变化并不大[11].我们也发现高Co含量对ODHP反应不利, 与文献结果一致.但通过以上实验也表明, 掺杂少量Co, 特别是Ni/Co摩尔比=9:1时, 有利于同时提高丙烷转化率和丙烯选择性, 这一发现未见相关文献报道, 其原因在下文中分析.表 2将我们的催化剂ODHP催化性能结果与文献中同类催化剂进行了对比, 3(Ni0.90Co0.10)Al MMO上丙烯收率明显优于文献值.
|
图 4
3(NixCo1-x)Al MMO催化剂的ODHP催化性能
Fig.4
Catalytic performances of 3(NixCo1-x)Al MMO catalysts for ODHP A: Propane conversion as a function of temperature, B: Propylene selectivity as a function of temperature.
(Reaction conditions: 350~550 ℃, C3H8:O2:N2= 2:2:16) 
|
| 表 2 3(Ni0.90Co0.10)Al MMO与文献同类催化剂的ODHP催化性能对比 Table 2 Comparison of the catalytic performance for the ODHP between (Ni0.90Co0.10)Al MMO and previously reported catalysts |
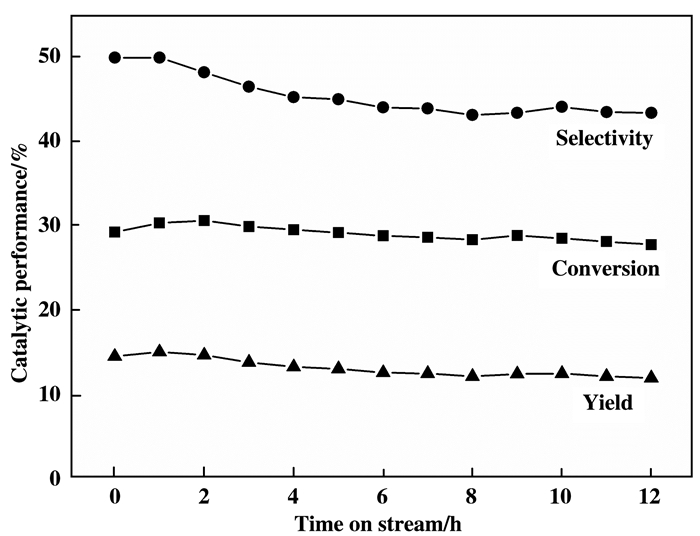
图 5为3(Ni0.90Co0.10)Al MMO催化剂连续反应的测试结果.由图可见, 450 ℃, VC3H8:VO2:VN2=2:2:16, GHSV= 6000 mL·h-1·g-1条件下, 反应12 h后3(Ni0.90Co0.10)Al MMO的丙烷转化率(28%)和丙烯选择性(44%)较初始值下降1%~5 %, 表明该催化剂在此反应条件下寿命稳定性较好.
|
图 5 3(Ni0.90Co0.10)Al MMO催化剂寿命测试 Fig.5 The lifetime test of 3(Ni0.90Co0.10)Al MMO catalyst (reaction conditions: T = 450 ℃, C3H8:O2:N2= 2:2:16, GHSV= 6000 mL·h-1·g-1) |
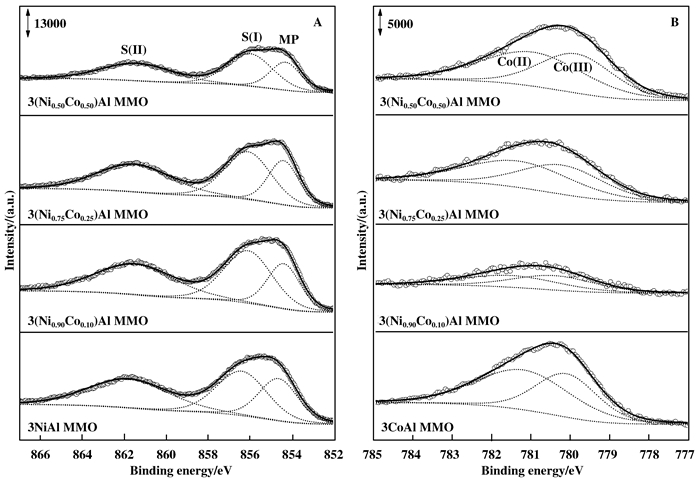
图 6为3(NixCo1-x)Al MMO催化剂的Ni 2p3/2(A)和Co 2p3/2(B) XPS谱图, 表 3为对应的XPS分析结果. 3NiAl MMO中854.7、856.4和861.8 eV处能谱峰分别为Ni(Ⅱ)自旋-轨道裂分2p3/2主峰(MP)和对应卫星峰S(Ⅰ)、S(Ⅱ)[22-23]. S(Ⅰ)与表面Ni2+空位或Ni3+相关, S(Ⅱ)与金属配体电荷转移有关[23]. S(Ⅰ)/MP峰强度比值与烯烃选择性存在相关性, 比值在1左右时, 相对大的比值有利于提高烯烃选择性[22]. 3CoAl MMO中780.1和781.2 eV处能谱峰分别为Co(Ⅲ)和Co(Ⅱ)的自旋-轨道裂分2p3/2主峰. Li Z等[24]研究发现, 具有尖晶石结构的Co(Ⅲ)物种有助于ODHP反应, Huang M X等[11]研究发现CoAl MMOs中Co(Ⅲ)/Co(Ⅱ)比值与层板和层间离子的主客体作用有关, 额外的Co(Ⅲ)可以促进氧活化和氧化还原循环.对3(NixCo1-x)Al MMO的XPS谱图分析不难发现, Ni(Ⅱ) 2p3/2的S(Ⅱ)/MP比值随Ni/Co比例基本无变化, 但3(Ni0.90Co0.10)Al MMO中S(Ⅰ)/MP和Co(Ⅲ)/Co(Ⅱ)比值较3NiAl MMO和3CoAl MMO均明显增大, 而其他3(NixCo1-x)Al MMO则变化相对较小, 这与其ODHP催化性能随Ni/Co比例的变化趋势一致.另外, 掺杂少量Co(x=0.90, 0.75)后使Ni(Ⅱ) 2p3/2主峰较3NiAl MMO向低结合能方向移动, 而Co(Ⅲ)和Co(Ⅱ) 2p3/2主峰较3CoAl MMO均向高结合能方向移动, 在3(Ni0.90- Co0.10)Al MMO中变化尤为明显, 表明表面Ni(Ⅱ)与Co(Ⅱ/Ⅲ)间通过Ni—O—Co键发生电子交互作用, 从而可能使S(Ⅰ)/MP和Co(Ⅲ)/Co(Ⅱ)比值增大.
|
图 6 3(NixCo1-x)Al MMO的Ni 2p3/2 (A)和Co 2p3/2(B) XPS谱图 Fig.6 XPS spectra of Ni 2p3/2 (A) and Co 2p3/2 (B) of 3(NixCo1-x)Al MMO |
| 表 3 (Ni0.90Co0.10)Al MMO催化剂的XPS分析结果 Table 3 XPS analysis of (Ni0.90Co0.10)Al MMO catalysts (eV) |
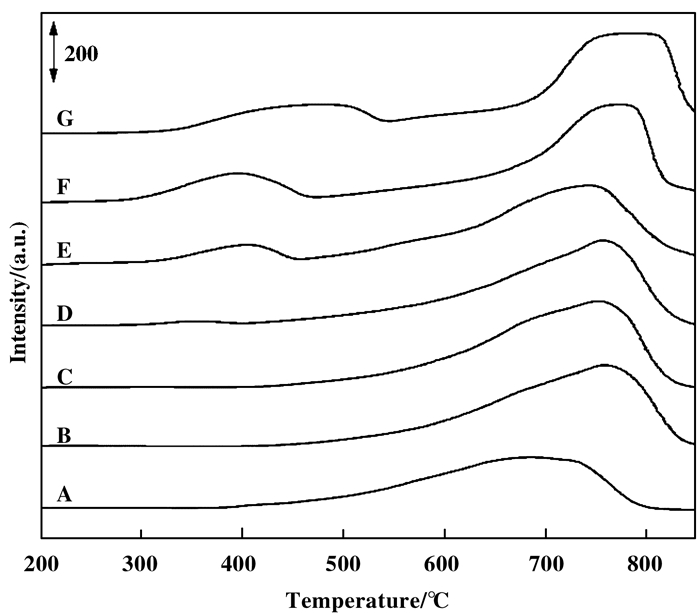
图 7为3(NixCo1-x)Al MMO催化剂的H2-TPR谱图, 对应分析结果列于表 4. 3NiAl MMO催化剂还原峰为一范围较宽的不对称峰(图 7A), 根据耗氢量将其归属于Ni(Ⅱ)Ni(0), 并可大致分为568 ℃低温还原峰, 对应表面非化学计量Ni(Ⅱ)物种还原; 666和735 ℃高温还原峰, 对应体相Ni-O和Ni-O-Al中Ni(Ⅱ)物种还原[25]. 3CoAl MMO催化剂呈现低温(350~550 ℃)和高温(550~900 ℃)两个还原阶段, 前者对应Co(Ⅲ)Co(Ⅱ), 后者对应Co(Ⅱ)Co(0)[11]. 3(NixCo1-x)Al MMO高温还原峰位均高于纯NiO或Co3O4, 这主要是惰性Al(Ⅲ)物种对M(Ⅱ)的分散作用所致, 亦表明表面金属氧化物分散性较高[8, 11].当Co含量较低(x ≥ 0.75)时, 3(Nix- Co1-x)Al MMO催化剂的H2-TPR谱图峰型(图 7B~D)与3NiAl MMO类似, 还原峰低温起始温度略有升高, 各还原峰位明显升高.在3(Ni0.75Co0.25)Al MMO中低温区出现微弱的Co(Ⅲ)Co(Ⅱ)还原峰.当Co含量继续增加至Ni/Co=1:1(x=0.50)时, Co(Ⅲ)Co(Ⅱ)低温还原峰明显可见(图 7E), 呈现与3CoAl MMO相似的双还原宽峰, 但还原峰位较3CoAl MMO均明显偏低50~60 ℃. x=0.25时, Co(Ⅲ)Co(Ⅱ)还原峰位受少量Ni(Ⅱ)影响仍偏低, 但高温还原峰已与3CoAl MMO接近(图 7F).整体而言, 随着Co含量增加, 高温还原峰位并未呈现逐渐升高趋势, 而是先升高, 再降低(x=0.50), 而后又升高.因此, 掺杂少量Co(x ≥ 0.75)后, 高温还原峰(主要源自Ni(Ⅱ)Ni(0))向高温方向移动可主要归因于少量Co掺杂增大样品比表面积, 金属离子分散性提高, 不易被H2还原, 而非因Co(Ⅱ)Co(0)还原温度较高所致.相反, Co含量较高(x≤0.50)时, 高温还原峰(主要源自Co(Ⅱ)Co(0))位置移动则应主要源于Co(Ⅱ)Co(0)还原温度较高.以上分析也可从高温还原峰位变化与催化剂比表面积(表 1)和晶相(图 2)变化规律的一致性上得到佐证.此外, 因Co存在变价, 且难以准确定量, 故对含Co样品并未通过耗氢量进行氧化态分析.但掺杂少量Co后, 催化剂耗氢量明显增大, 3(Ni0.90Co0.10)Al MMO尤为显著, 表明其中Co(Ⅲ)含量较Co(Ⅱ)高, 且可能存在Ni(Ⅲ), 这与XPS表征结果一致.
|
图 7 3(NixCo1-x)Al MMO催化剂的H2-TPR谱图 Fig.7 H2-TPR profiles of 3(NixCo1-x)Al MMO catalysts A~G: x=1, 0.95, 0.90, 0.75, 0.50, 0.25, 0 |
| 表 4 3(NixCo1-x)Al MMO催化剂的H2-TPR分析结果 Table 4 H2-TPR analysis of (NixCo1-x)Al MMO catalysts |
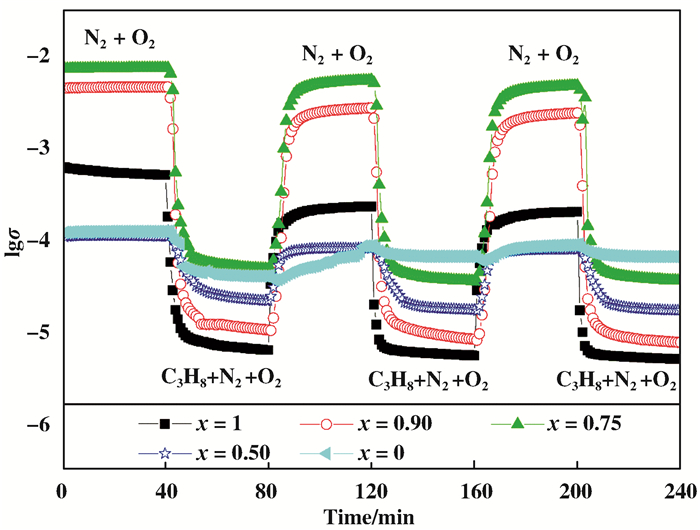
图 8为3(NixCo1-x)Al MMO在不同气氛中的原位电导率变化图.在N2+O2气氛中p型半导体氧化物催化剂空穴增加, 被氧化, 电导率随接触时间先增加后稳定; 通入烷烃后, 消耗空穴, 催化剂被还原, 电导率立即减小, 之后可达到稳定值; 再次切换上述气氛, 若此过程可重复出现, 表明催化剂表面晶格O-物种(催化活性位)可循环再生, 氧化还原可逆性良好[26], 这与ODH氧化还原机理, 即Mars-van Krevelen机理[27]一致.由图 8可见, 3NiAl MMO和掺杂少量Co(x≥0.75)的3(NixCo1-x)Al MMO催化剂的原位电导率随气氛变化循环规律变化, 即氧化还原可逆性均良好; 而掺杂大量Co的3(Ni0.50Co0.50)Al MMO和3CoAl MMO氧化还原可逆性差.此外, Δ[lg(σ)]表示在不同气氛中稳态电导率的差值, 被认为是在C3H8+N2+O2气氛相对于N2+O2气氛从催化剂中去除表面晶格氧物种数目的量度, 即相对较大的Δ[lg(σ)], 表明催化剂表面晶格氧物种数较多, 低碳烷烃ODH催化活性增强[18].由图 8可见, Δ[lg(σ)]按如下顺序减小: 3(Ni0.90Co0.10)Al MMO (2.6) > 3(Ni0.75Co0.25)Al MMO (1.9) > 3NiAl MMO (1.6) > 3(Ni0.50Co0.50)Al MMO (0.7) > 3CoAl MMO (0.5).这与其ODHP催化性能一致.
|
图 8 450 ℃时3(NixCo1-x)Al MMO在N2+O2和C3H8+N2+O2气氛下的原位电导率变化图 Fig.8 Variation of the in-situ electrical conductivity of 3(NixCo1-x)Al MMO during sequential exposures to N2+O2 and C3H8+N2+O2 stream at 450 ℃ (σ in Ω-1 cm-1) (x= 1, 0.90, 0.75, 0.50, 0) |
综上所述, 以不同Ni/Co比例的3(NixCo1-x)Al LDH前驱体600 ℃焙烧得3(NixCo1-x)Al MMO催化剂, 其表面织构、晶相和形貌随Ni/Co比例规律变化. ODHP催化性能测试结果表明, 掺杂大量Co对反应不利, 而掺杂少量Co的3(Ni0.90Co0.10)Al MMO上催化活性最佳:丙烯选择性随反应温度升高降低不明显, 500 ℃时丙烷转化率31%, 丙烯选择性48%, 丙烯收率15%.分析表明, 将少量Co引入3NiAl LDH前驱体替代部分Ni(Ni/Co摩尔比=9:1), 焙烧后获得的3(Ni0.90Co0.10)Al MMO表面Ni(Ⅱ)与Co(Ⅱ/Ⅲ)间存在明显电子交互作用, 类NiO相分散性提高, 表面晶格氧物种增多, 具有优良的氧化还原可逆性.以上实验表明, 在3(NixCo1-x)Al MMO中, Ni(Ⅱ)与少量掺杂的Co(Ⅱ/Ⅲ)间存在协同作用, 在恰当比例时可展现出良好的ODHP催化活性, 但其相互作用的根本机制仍需深入研究.
| [1] |
a. Fan X, Liu D, Zhao Z, et al. Influence of Ni/Mo ratio on the structure-performance of ordered mesoporous Ni-Mo-O catalysts for oxidative dehydrogenation of propane[J]. Catal Today, 2020, 339 : 67-78. b. Wang L, Chu W, Jiang C, et al. Oxidative dehydrogenation of propane over Ni-Mo-Mg-O catalysts[J]. J Nat Gas Chem, 2012, 21 (1): 43-48. |
| [2] |
a. Sattler J J H B, Ruiz-Martinez J, Santillan-Jimenez E, et al. Catalytic dehydrogenation of light alkanes on metals and metal oxides[J]. Chem Rev, 2014, 114 (20): 10613-10653. b. Grabowski R. Kinetics of oxidative dehydrogenation of C2-C3 alkanes on oxide catalysts[J]. Catal Rev, 2006, 48 (2): 199-268. c. Zhang Qiao (张巧), Zhang Ke-ting (张客厅), Wang Chen-guang (王晨光), et al. The performance of propane dehydrogenation over PtSn metal loaded on calcined Mg-Al layered double hydrotalcite (负载PtSn金属助剂的镁铝水滑石上的丙烷脱氢反应研究)[J]. J Mol Catal (China) (分子催化), 2018, 32 (4): 359-369. d. Zhang Ya-liu (张亚柳), Yang Shuang (杨双), Shi Mian (师勉), et al. Preparation of Cr/MCM-41 catalyst and its application for nonoxidative dehydrogenation of propane (Cr/MCM-41催化剂的制备及其用于丙烷非氧化脱氢反应)[J]. J Mol Catal (China) (分子催化), 2018, 32 (5): 425-433. |
| [3] |
a. Védrine J C, Fechete I. Heterogeneous partial oxidation catalysis on metal oxides[J]. C R Chimie, 2016, 19 (10): 1203-1225. b. Li Z, Peters A W, Platero-Prats A E, et al. Fine-tuning the activity of metal-organic framework-supported cobalt catalysts for the oxidative dehydrogenation of propane[J]. J Am Chem Soc, 2017, 139 (42): 15251-15258. c. Sun M, Zhang J, Putaj P, et al. Catalytic oxidation of light alkanes (C1-C4) by heteropoly compounds[J]. Chem Rev, 2013, 114 (2): 981-1019. |
| [4] |
Guo Jian-ping(郭建平), Yi Xiao-dong(伊晓东), Wu Zhong-fang(吴钟芳), et al. Effect of cesium loading on catalytic activity of VOx/SBA-15 catalysts for propane oxidative dehydrogenation(铯添加对VOx/SBA-15催化剂丙烷氧化脱氢性能影响)[J]. J Mol Catal (China)(分子催化), 2011, 25(5): 415–420.
|
| [5] |
Qi W, Su D S. Metal-free carbon catalysts for oxidative dehydrogenation reactions[J]. ACS Catal, 2014, 4(9): 3212–3218.
DOI:10.1021/cs500723v |
| [6] |
a. Grant J T, Carrero C A, Goeltl F, et al. Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts[J]. Science, 2016, 354 (6319): 1570-1573. b. Yan B, Li W C, Lu A H. Metal-free silicon boride ca- talyst for oxidative dehydrogenation of light alkanes to olefins with high selectivity and stability[J]. J Catal, 2019, 369 : 296-301. |
| [7] |
Valverde J A, Echavarría A, Eon J G, et al. V-Mg-Al catalyst from hydrotalcite for the oxidative dehydrogenation of propane[J]. Reac Kinet Mech Catal, 2014, 111(2): 679–696.
DOI:10.1007/s11144-014-0674-6 |
| [8] |
Smoláková L, Capek L, Botková Š, et al. Activity of the Ni-Al mixed oxides prepared from hydrotalcite-like precursors in the oxidative dehydrogenation of ethane and propane[J]. Top Catal, 2011, 54(16/18): 1151–1162.
|
| [9] |
Valverde J A, Echavarría A, Ribeiro M F, et al. Decavanadate-intercalated Ni-Al hydrotalcites as precursors of mixed oxides for the oxidative dehydrogenation of propane[J]. Catal Today, 2012, 192(1): 36–43.
DOI:10.1016/j.cattod.2012.04.043 |
| [10] |
Mitran G, Cacciaguerra T, Loridant S, et al. Oxidative dehydrogenation of propane over cobalt-containing mixed oxides obtained from LDH precursors[J]. Appl Catal A: Gen, 2012, 417: 153–162.
|
| [11] |
Huang M X, Wu X, Yi X D, et al. Highly dispersed CoOx in layered double oxides for oxidative dehydrogenation of propane: guest-host interactions[J]. RSC Adv, 2017, 7(24): 14846–14856.
DOI:10.1039/C7RA01190C |
| [12] |
Álvarez M G, Urd A, Rives V, et al. Propane oxidative dehydrogenation over V-containing mixed oxides derived from decavanadate-exchanged ZnAl-layered double hydroxides prepared by a sol-gel method[J]. C R Chimie, 2018, 21(3/4): 210–220.
|
| [13] |
a. Xu M, Wei M. Layered double hydroxide-based catalysts: Recent advances in preparation, structure, and applications[J]. Adv Funct Mater, 2018, 28 (47): 1802943. b. Zhu Lin-hua (祝琳华), Li Feng-long (李奉隆), Si Tian (司甜), et al. Preparation of layered clay-supported gold catalysts and catalytic activity for CO oxidation at room temperature (层状粘土负载的金催化剂制备及其常温催化氧化活性)[J]. J Mol Catal (China) (分子催化), 2016, 30 (1): 46-53. |
| [14] |
Sun Y F, Li G C, Pan X D, et al. Oxidative dehydrogenation of propane to propylene over mesoporous alumina supported Ni-Co oxide catalysts[J]. Acta Phys Chim Sin, 2012, 28(9): 2135–2140.
|
| [15] |
Cai T, Yuan J, Zhang L, et al. Ni-Co-O solid solution dispersed nanocrystalline Co3O4 as a highly active catalyst for low-temperature propane combustion[J]. Catal Sci Technol, 2018, 8(21): 5416–5427.
DOI:10.1039/C8CY01062E |
| [16] |
a. Zhaorigetu B, Li W Z, Xu H Y, et al. Correlation between the characteristics and catalytic performance of Ni-V-O catalysts in oxidative dehydrogenation of propane[J]. Catal Lett, 2004, 94 (1/2): 125-129. b. Li T, Wang J, Zhaorigetu B, et al. Effect of preparation parameters on the catalytic performance of mesoporous NiO for the oxidative dehydrogenation of propane to propylene[J]. Reac Kinet Mech Catal, 2013, 110 (2): 421-435. |
| [17] |
Lv Zhi(吕志), Duan Xue(段雪). Controllable preparation of layered anionic materials(阴离子层状材料的可控制备)[J]. Chin J Catal(催化学报), 2008, 29(9): 839–856.
DOI:10.3321/j.issn:0253-9837.2008.09.005 |
| [18] |
Ivan Ç B, Popescu I, Fechete I, et al. The effect of phosphorus on the catalytic performance of nickel oxide in ethane oxidative dehydrogenation[J]. Catal Sci Technol, 2016, 6(18): 6953–6964.
DOI:10.1039/C6CY00946H |
| [19] |
Thommes M, Kaneko K, Neimark A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure Appl Chem, 2015, 87(9/10): 1051–1069.
|
| [20] |
Kovanda F, Rojka T, Bezdicka P, et al. Effect of hydrothermal treatment on properties of Ni-Al layered double hydroxides and related mixed oxides[J]. J Solid State Chem, 2009, 182(1): 27–36.
DOI:10.1016/j.jssc.2008.09.014 |
| [21] |
Rotan M, Tolchard J, Rytter E, et al. On the solid solution of the spinel phase in the system NiO-Al2O3[J]. J Solid State Chem, 2009, 182(12): 3412–3415.
DOI:10.1016/j.jssc.2009.10.001 |
| [22] |
a. Solsona B, Nieto J M L, Concepción P, et al. Oxidative dehydrogenation of ethane over Ni-W-O mixed metal oxide catalysts[J]. J Catal, 2011, 280 (1): 28-39. b. Solsona B, Concepción P, Demicol B, et al. Selective oxidative dehydrogenation of ethane over SnO2-promoted NiO catalysts[J]. J Catal, 2012, 295 : 104-114. c. Heracleous E, Lee A F, Wilson K, et al. Investigation of Ni-based alumina-supported catalysts for the oxidative dehydrogenation of ethane to ethylene: structural characterization and reactivity studies[J]. J Catal, 2005, 231 (1): 159-171. |
| [23] |
a. Biju V, Khadar M A. Electronic structure of nanostructured nickel oxide using Ni 2p XPS analysis[J]. J Nanopart Res, 2002, 4 (3): 247-253. b. Van Veenendaal M A, Alders D, Sawatzky G A. Influence of superexchange on Ni 2p x-ray-absorption spectroscopy in NiO[J]. Phys Rev B, 1995, 51 (20): 13966-13971. c. Alders D, Voogt F C, Hibma T, et al. Nonlocal screening effects in 2p x-ray photoemission spectroscopy of NiO (100)[J]. Phys Rev B, 1996, 54 (11): 7716-7719. d. Van Veenendaal M A, Sawatzky G A. Nonlocal screening effects in 2p x-ray photoemission spectroscopy core-level line shapes of transition metal compounds[J]. Phys Rev Lett, 1993, 70 (16): 2459-2462. |
| [24] |
Li Z, Peters A W, Bernales V, et al. Metal-organic framework supported cobalt catalysts for the oxidative dehydrogenation of propane at low temperature[J]. ACS Cent Sci, 2016, 3(1): 31–38.
|
| [25] |
Wang J, Lang X, Zhaorigetu B, et al. Aerobic oxidation of alcohols on Au nanocatalyst: Insight to the roles of the Ni-Al layered double hydroxides support[J]. ChemCatChem, 2014, 6(6): 1737–1747.
DOI:10.1002/cctc.201400046 |
| [26] |
a. Popescu I, Heracleous E, Skoufa Z, et al. Study by electrical conductivity measurements of semiconductive and redox properties of M-doped NiO (M= Li, Mg, Al, Ga, Ti, Nb) catalysts for the oxidative dehydrogenation of ethane[J]. Phys Chem Chem Phys, 2014, 16 (10): 4962-4970. b. Popescu I, Skoufa Z, Heracleous E, et al. A study by electrical conductivity measurements of the semiconductive and redox properties of Nb-doped NiO catalysts in correlation with the oxidative dehydrogenation of ethane[J]. Phys Chem Chem Phys, 2015, 17 (12): 8138-8147. |
| [27] |
Herrmann J M, Vernoux P, Béré K E, et al. In situ study of redox and of p-Type semiconducting properties of vanadyl pyrophosphate and of V-P-O catalysts during the partial oxidation of n-butane to maleic anhydride[J]. J Catal, 1997, 167(1): 106–117.
DOI:10.1006/jcat.1997.1537 |
 2019, Vol. 33
2019, Vol. 33


