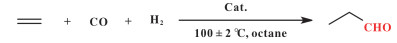2. University of Chinese Academy of Sciences, No. 19 A, Yuquanlu, Beijing 100049, China;
3. Dalian National Laboratory for Clean Energy, Dalian, 116023, China
2. 中国科学院大学, 北京, 100049;
3. 大连洁净能源国家实验室, 辽宁 大连, 116023
Hydroformylation of olefins is one of the most momentous industrial processes for the synthesis of aldehyde, which provides more than 10 million tons' annual output[1-3]. The aldehyde can be extensively served as key intermediates in the preparation of esters, alcohols, carboxylic acids, aliphatic amines, and other fine chemicals[4-5]. Among the hydroformylation reactions, ethylene hydroformylation, which can generate propanal and 1-propanol, plays an important role. Nowadays, ligand-modified Rh-based homogeneous catalysts are widely used in industrial processes because of their remarkable activity and selectivity as well as mild reaction conditions[5-6]. However, this Rh-based homogeneous process faces the problem of losing catalytic active species, catalyst separation and recovery. Moreover, the use of organic ligand makes the operation complicated. Compared with homogeneous catalysts, heterogeneous counterparts can be more facilely separated and recycled. Thus, extensive efforts have been devoted to exploit heterogeneous catalysts by loading Rh nanoparticles onto solid supports. So far, Rh supported on many solid supports, such as SiO2[7-10], carbon materials[7, 11-14], TiO2[15], Al2O3[16-17], Cu2O[18], ZnO[19], zeolite[20-22], and MOF[23-25], have been investigated for heterogeneous ethylene hydroformylation. Nevertheless, their activities and stability are usually low compared with the corresponding homogeneous catalysts[26]. To enhance the activity and stability of the heterogeneous catalyst, a nice method of organic phosphine ligand functionalized support via ligand immobilization or polymerization was developed and applied for the preparation of heterogeneous hydroformylation catalysts[27-32]. However, the use of expensive and air sensitive phosphine ligand limited the potential practical applications. So far, the report of highly efficient and stable heterogeneous catalysts without using phosphine ligand toward hydroformylation is still rare.
In fact, it has been reported that the coordination of O-containingligands onto Rh, such as Rh(acac)(CO)2, can result in high activity for hydroformylation by homogeneous catalysts[33]. Therefore, it should be possible to design an active and stable phosphine ligand-free heterogeneous catalyst for ethylene hydroformylation. Among various supports, activated carbons have the advantage of adjustable surface O-containing functional groups. Therefore, we envision that activated carbon with suitable O-coordinating surface functional groups could serve as a good support candidate to prepare highly efficient, stable, and phosphine ligand-free heterogeneous hydroformylation catalysts (Fig. 1). However, although carbon materials as supports have been explored for long time[7, 11-12, 14], the detailed investigation between the surface structure of carbon materials and catalytic performance is rarely involved.
|
Fig.1 (a) Rh/POL-PPh3 (POL-PPh3:3V-PPh3 polymer)[34]; (b) Rh(acac)(CO)2[33]; (c) Rh/C catalyst including air stable O-containing functional groups |
Based on our extensively studies on catalyticcarbonylation with CO, CO2 and other carbonyl resources[35-42], herein, we reported that an activated carbon, which mainly contains lactone and carboxylic acid groups, can be an ideal support for active and stable ethylene hydroformylation catalyst prepara- tion.
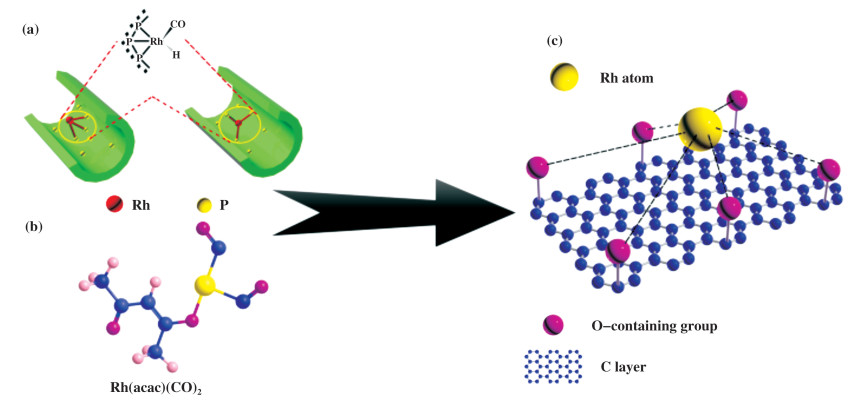
1 Results and discussion 1.1 Characterization of catalystsFirst, a series of activated carbons with different O-containing functional groups selected, in which the surface oxygenated groups of the activated carbon supports were explored by Boehm titration analysis[43]. Finally, four commercially available activated carbons, i.e., C-1 to C-4, were chosen (Fig. 2(a)). Based on the Boehm titration analysis, the ketone, hydroxyl, lactone and carboxylic groups on C-1, C-3, and C-4 are 0, 309, 10, 70; 0, 0, 624, 276; and 1, 231, 201, 501, 461 μmol/g, respectively. No surface oxygenated groups were detectable for C-2.
|
Fig.2 Structure of the catalysts. Contents of surface oxygenated groups by Boehm titration (a), HR-TEM image of Rh/C-1 (b), STEM of Rh/C-1(c), Rh/C-2 (d), Rh/C-2 (e), Rh/C-3 (f), Rh/C-3 (g), Rh/C-4 (h), Rh/C-4 (i) Insets are the corresponding particle size distributions |
The supported Rh catalysts were prepared by the precipitation-deposition method and characterized by TEM, XRD, XPS and N2 adsorption-desorption analyses to reveal their structures. First, the morphology and size of Rh NPs in the Rh/C samples were characterized by TEM, and the results are shown in Fig. 2. The STEM images of the Rh/C-1 and Rh/C-2 samples exhibit a large mean particle size of 5.0 and 5.6 nm with a broad particle size distribution from 2 to 10 nm due to the aggregation of the NPs (Fig. 2(c), (e)). Notably, the STEM images of fresh and used Rh/C-3 show that the Rh NPs are well dispersed on C-3 and exhibit a small mean particle size of 2.2 and 2.1 nm with a narrow particle size distribution from 1.5 to 3 nm, which might be attributed to the fact that the functional groups on C-3 can be used for anchoring the Rh nanoparticles (Fig. 2(g)). The HR-TEM images of Rh particles on fresh and used Rh/C-3 show a lattice spacing of 0.22 nm, which can be indexed to the (111) plane of Rh. Based on above results, we suppose that the lactone group on the surface of C-3 plays a critical role in generating highly dispersed and small Rh NPs.
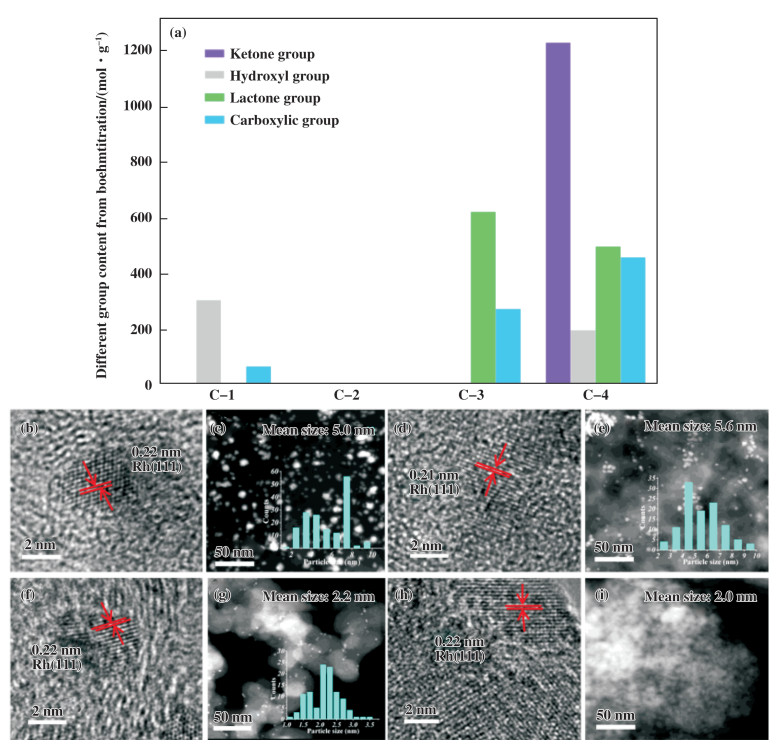
The XRD patterns of differentRh/C and used Rh/C-3 samples showed that no peak corresponded to the Rh species can be observed in all the catalysts, which is probably attributed to the small Rh particle size or that the Rh species were amorphous. Moreover, XPS studies of the catalysts were performed to determine the chemical state of Rh nanoparticles. As shown in Fig. 3, the XPS spectra of Rh 3d in Rh/C-1 and Rh/C-2 show two peaks located at binding energies of 307.6 and 312.5 eV, corresponding to the metallic Rh 3d5/2 and Rh 3d3/2, respectively. The Rh 3d signals of Rh/C-3, Rh/C-4, and Rh/C-3 after being used were not observed, which might be attributed to low Rh loadings. The N2 adsorption-desorption tests showed that the BET surface areas of the catalysts of Rh/C-1, Rh/C-2, Rh/C-3, and Rh/C-4 were 1423, 408, 191 and 1607 m2/g, in which the BET surface area of Rh/C-3 is the lowest. Therefore, the better catalytic performance of Rh/C-3 should not be attributed to the surface area.
|
Fig.3 XPS spectra of the Rh/C catalysts (from top: Rh/C-1, Rh/C-2, Rh/C-3, Rh/C-4 and Rh/C-3 after being used) |
Then, the catalytic performance of the Rh/C catalysts was tested (Table 1). Initial hydroformylation experiments were performed at 6 MPa syngas (CO/H2 = 1:1) with octane as solvent. Clearly, Rh/C-3 exhibits the best catalytic performance. The turnover frequency (TOF) of ethylene reached 3436 h-1 and the desired hydroformylation product, i.e., propanal, was obtained in 64% yield (Entry 1-4, Table 1). It should be noted that lower conversions were observed in case of other Rh/C (Rh/C-1, Rh/ C-2, and Rh/ C-4) were employed as catalysts. Based on the Rh loading of different Rh/C catalysts, the TOF for propanal formation of Rh/C-1, Rh/C-2, Rh/C-3 and Rh/C-4 are 1897, 1768, 3436, and 1168 h-1, respectively (Entries 1-4). Thus, the order of catalytic performance of the different Rh/C catalysts is Rh/C-3 > Rh/C-1 > Rh/C-2 > Rh/C-4. It is clear that the catalytic performances of the Rh/C are strongly dependent on the structures and quantities of surface oxygenated groups. Combined the Boehm titration analysis and TEM results, we could draw a conclusion that ketone group on the surface of C-3 might play a critical role in highly dispersed and smaller Rh NPs formation, thus resulting in higher catalytic activity of Rh/C-3 for hydroformylation reaction. The controlled experiment indicated that the RhCl3·XH2O as catalyst showed a trace conversion of ethylene under the same conditions (Entry 5). In addition, physical mixtures of RhCl3·XH2O and activated carbons were explored (Entries 6-9). Interesting, relatively good yield of 43% and TOF of 2004 h-1 were obtained with physical mixtures of RhCl3·XH2O and C-3 as catalyst, while almost no proponal was observed when C-1, C-2, and C-4 were employed (Entries 6-9). This is a strong evidence to support that C-3 is crucial in the construction of active hydroformylation catalyst. As is well known, [Rh(COD)Cl]2 and Rh(CO)(acac)2 are classical catalyst precursors in hydroformylation of alkenes[44-45]. If [Rh(COD)Cl]2 was employed, much lower TOF of 2491 h-1 was obtained at the same reaction conditions (Entry 10). Meanwhile, the hydroformylation with Rh(CO)(acac)2 catalyst shows a high TOF of 3792 h-1 (Entry 11). The above results demonstrated the comparable activity of the heterogeneous Rh/C-3. Notably, much higher turnover frequency (TOF) of 57 889 h-1 was achieved by decreasing the loadings of Rh/C-3 catalyst and increasing the quantity of ethylene, which was 5 times of the highest active homogeneous catalyst (TOF up to 10 627 h-1) in previous study (Entry 12)[46].
| Table 1 Screening of the catalyst for ethylene hydroformylationa |
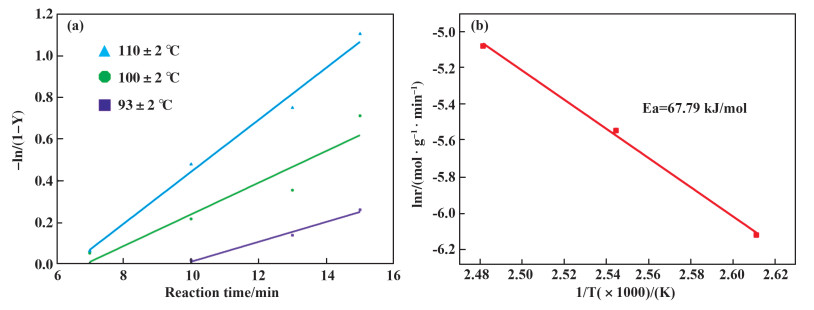
Furthermore, the kinetic study on the hydroformylation of ethylene to propanal was conducted over Rh/C-3 catalyst. The evolution relationship of ethylene conversion with reaction time at different temperatures over Rh/C-3 catalyst was investigated (Fig. 4(a)). An Arrhenius plot for ethylene hydroformylation in the temperature interval 93~110 ℃ is obtained, and the apparent activation energy for n-propanal formation was calculated to be 67.79 kJ·mol-1 (Fig. 4(b)).
|
Fig.4 (a). Effect of temperature on rate of propanal synthesis. Ethylene (5 mmol), Rh/C-3 (25 mg, 0.0010 mmol Rh), CO (3 MPa), H2 (3 MPa), octane (5.0 mL); (b). Arrhenius plot for apparent activation energies of the Rh/C-3 catalyst |
The stability of the catalyst is very important in heterogeneous catalysis. Therefore, the stability of the Rh/C-3 catalyst in the hydroformylation of ethylene was investigated with flow system, which would be more valuable in practical process (Fig. 5). To our delight, the propanal yield remained stable for over 2500 h, and the selectivity for propanal was >99.7%, with the generation of trace amounts of ethane, clearly demonstrating that the catalyst was extremely stable. The Rh loadings of the fresh and used Rh/C-3 catalysts over 2500 h were all in the range of 0.4%~0.5%(Percent weight). Therefore, the Rh/C-3 catalyst is stable enough in the fixed-bed system, and hydroformylation with a heterogeneous Rh catalyst was realized. In previous reports, the lifetime of phosphine ligand-free heterogeneous catalysts was ~70 h[47], while the number is ~1000 h[34] with supported phosphine ligand. Thus, our catalyst is the most stable heterogeneous catalyst for ethylene hydroformylation in flow reaction systems so far.

|
Fig.5 Stability test of the Rh/C-3 catalyst for ethylene hydroformylation Reaction conditions: a fixed-bed reactor, P=6.0 MPa (C2H4:CH4:CO:H2 =1:1:24:24), T = 95 ℃, 2983 mL/g Cat/h. CH4 was used as the internal standard material for calibration |
In conclusion, we have demonstrated a nice heterogeneous ethylenehydroformylation catalyst with both excellent activity and stability based on phosphine ligand free Rh/C-3 catalyst by replacing the phosphine ligand with air-stable O-containing functional groups on the activated carbon surface. The Rh/C-3 catalyst showed excellent performance in converting ethylene to propanal with high activity and selectivity. Notably, the Rh/C-3 catalyst exhibited substantially improved stability in the hydroformylation of ethylene in a fixed-bed reactor (at least 2500 h). The Boehm titration experiments revealed that the excellent performance of this Rh/C-3 catalyst could be attributed to that the presence of lactone group (-CO2-) on the surface of activated carbon. It not only facilitates the uniform distribution and stabilization of Rh nanoparticles but also boosts the catalytic activity. The results described here may promote the design of active, stable and organic phosphine free heterogeneous catalysts for alkene hydroformylation.
Experiments General InformationXRD measurements were conducted by using a Empyrean instrument equipped with a ceramic X-ray tube and maximum power of 2.2 kW. The XRD patterns were scanned in the 2 Theta range of 10°~90°. XPS were obtained using a Escalab 250Xi instrument equipped with monochromatic Al target and dual anode Al/Mg target X-ray sources, including large-area XPS, micro-area XPS and XPS parallel imaging. The electron binding energy was referenced to the C 1s peak at 284.8 eV. The background pressure in the chamber was less than 10-13 MPa. TEM was carried out by using a Tecnai G2 F30 S-Twin transmission electron microscope operating at 300 kV. For TEM investigations, the catalysts were dispersed in ethanol by ultrasonication and deposited on carbon-coated copper grids. The BET surface area measurements were performed on a Quantachrome IQ2 at the temperature of -196 ℃. The pore size distribution wascalculated from the desorption isotherm by using the Barrett, Joyner, and Halenda (BJH) method.
Rh/C Catalysts Preparation500 mg C were added into a 100 mL round-bottom flask with 50 mL distilled water, and dispersed by ultrasound for 30 min. Then 1 mL RhCl3·XH2O (5 mg Rh/mL) was added, and further stirred for 4 h at room temperature. Then the pH was adjusted by dropwise addition of an aqueous solution of NaOH (2 mol/L), and reduced by dropwise addition of 3 mL formaldehyde (37%~40% H2O) in 80 ℃ oil bath for 2 h. The precipitates were washed with deionized water, dried in the 80 ℃ oven for 12 h. Next, the mixture of the solid and octane were added into a glass tube in 160 ℃ for 48 h. Finally, the precipitates were washed with ethyl acetate, and dried in the 80 ℃ oven for 12 h.
Typical procedure for hydroformylation of ethyleneA mixture of ethylene (5 mmol), 1%(Percent weight)Rh/C-3 (25 mg), and octane (5 mL) were added into a glass tube which was placed in an 80 mL autoclave. Then the autoclave was purged and charged with CO (3 MPa) and H2 (3 MPa). The reaction mixture was stirred at 120 ℃ for 3 h. After the reaction finished, the autoclave was cooled to room temperature and the pressure was carefully released. Subsequently, the reaction mixture was diluted with 5 mL of ethyl acetate. After the solid catalyst was filtered from the solution, the liquid products were analyzed quantitatively by a gas chromatograph (Agilent 7890A) equipped with a capillary column (HP-5) and a FID detector with decane as an internal standard.
Typical procedures of flow reactions with fix-bed reactorThe hydroformylation of ethylene was conducted in a continuous flow fixed-bed reactor with an inner diameter of 6 mm at 95 ℃, 6 MPa syngas. In catalytic test, 1 g of catalyst was charged into a stainless steel tubular reactor. The effluent was passed through a 500 mL round-bottom flask filled with 300 mL of ethyl acetate and the round-bottom flask was cooled to -20 ℃ by a condenser. Propanal was captured by dissolution into the ethyl acetate in the flask. Then, The ethyl acetate solution containing propanal was analyzed quantitatively by a gas chromatograph (Agilent 7890A) with decane as an internal standard, and the gaseous substrate stream was collected by an airbag and monitored by an off-line gas chromatograph from Shimadzu with a FID detector (50 m 19095P-S25 column at 100 ℃).
The calculated way for plotting Figure 3 from the rate constant k is as follows:The corresponding natural logarithm (lnk) values of the rate constants (k) at different temperatures from 93 to 110 ℃ for the catalysts were calculated firstly. Then, based on the Arrhenius equation: lnk = lnA-Ea/RT (logarithmic expression), the plots of lnk~1/T using linear regression fitting in Origin 8.0 for the Rh/C-3 catalysts were obtained. The corresponding coefficients of determination of the linear regression fitting for Rh/C-3 is R2 = 0.998.
| [1] |
Piet W N M, Van Leeuwen, Claver C. Rhodium catalyzed hydroformylation[M]. Netherlands: Kluwer Academic Publishers, 2002: 1-13.
|
| [2] |
Arpe H J. Industrial organic chemistry[M]. Germany: Wiley-VCH, 2010.
|
| [3] |
Breit B, Seiche W. Recent advances on chemo-, regio-and stereoselective hydroformylation[J]. Synthesis, 2001, 2001(1): 1–36.
|
| [4] |
Franke R, Selent D, Borner A. Applied hydroformylation[J]. Chem Rev, 2012, 112(11): 5675–5732.
DOI:10.1021/cr3001803 |
| [5] |
Wu X F, Fang X, Wu L, et al. Transition-metal-catalyzed carbonylation reactions of olefins and alkynes:A personal account[J]. Accounts Chem Res, 2014, 47(4): 1041–1053.
DOI:10.1021/ar400222k |
| [6] |
Beller M, Cornils B, Frohning C D, et al. Progress in hydroformylation and carbonylation[J]. J Mol Catal A:Chem, 1995, 104(1): 17–85.
DOI:10.1016/1381-1169(95)00130-1 |
| [7] |
Sakagami H, Ohta N, Endo S, et al. Location of active sites for 3-pentanone formation during ethene hydroformylation on Rh/active-carbon catalysts[J]. J Catal, 1997, 171(2): 449–456.
|
| [8] |
Sandee A J, Reek J N, Kamer P C, et al. A silica-supported, switchable, and recyclable hydroformylation-hydrogenation catalyst[J]. J Am Chem Soc, 2001, 123(35): 8468–8476.
DOI:10.1021/ja010150p |
| [9] |
Borrmann T, McFarlane A J, Ritter U, et al. Rhodium catalysts build into the structure of a silicate support in the hydroformylation of alkenes[J]. Cent Eur J Chem, 2013, 11(4): 561–568.
|
| [10] |
Marchetti M, Paganelli S, Viel E. Hydroformylation of functionalized olefins catalyzed by SiO2-tethered rhodium complexes[J]. J Mol Catal A:Chem, 2004, 222(1/2): 143–151.
|
| [11] |
Li B T, Li X H, Asami K, et al. Hydroformylation of 1-hexene over rhodium supported on active carbon catalyst[J]. Chem Lett, 2003, 32(4): 378–379.
DOI:10.1246/cl.2003.378 |
| [12] |
Kainulainen T A, Niemela M K, Krause A O I. Hydroformylation of 1-hexene on Rh/C and Co/SiO2 catalysts[J]. J Mol Catal A:Chem, 1997, 122(1): 39–49.
DOI:10.1016/S1381-1169(96)00461-X |
| [13] |
Li X, Zhang Y, Meng M, et al. Silicalite-1 membrane encapsulated Rh/activated-carbon catalyst for hydroformylation of 1-hexene with high selectivity to normal aldehyde[J]. J Membr Sci, 2010, 347(1): 220–227.
|
| [14] |
Ganga V S R, Dabbawala A A, Munusamy K, et al. Rhodium complexes supported on nanoporous activated carbon for selective hydroformylation of olefins[J]. Catal Commun, 2016, 84: 21–24.
DOI:10.1016/j.catcom.2016.05.021 |
| [15] |
Shi Y, Hu X, Chen L, et al. Boron modified TiO2 nanotubes supported Rh-nanoparticle catalysts for highly efficient hydroformylation of styrene[J]. New J Chem, 2017, 41(14): 6120–6126.
DOI:10.1039/C7NJ01050H |
| [16] |
Navidi N, Thybaut J W, Marin G B. Experimental investigation of ethylene hydroformylation to propanal on Rh and Co based catalysts[J]. Appl Catal A-Gen, 2014, 469: 357–366.
DOI:10.1016/j.apcata.2013.10.019 |
| [17] |
Alini S, Bottino A, Capannelli G, et al. Preparation and characterisation of Rh/Al2O3 catalysts and their application in the adiponitrile partial hydrogenation and styrene hydroformylation[J]. Appl Catal A-Gen, 2005, 292: 105–112.
DOI:10.1016/j.apcata.2005.05.048 |
| [18] |
Jagtap S A, Bhosale M A, Sasaki T, et al. Rh/Cu2O nanoparticles:Synthesis, characterization and catalytic application as a heterogeneous catalyst in hydroformylation reaction[J]. Polyhedron, 2016, 120: 162–168.
DOI:10.1016/j.poly.2016.08.026 |
| [19] |
Kontkanen M L, Tuikka M, Kinnunen N, et al. Hydroformylation of 1-hexene over Rh/nano-oxide catalysts[J]. Catalysts, 2013, 3(1): 324–337.
DOI:10.3390/catal3010324 |
| [20] |
Davis M E, Rode E, Taylor D, et al. A comparison of X-zeolite-supported and Y-zeolite-supported rhodium as propylene hydroformylation catalysts[J]. J Catal, 1984, 86(1): 67–74.
|
| [21] |
Rode E J, Davis M E, Hanson B E. Propylene hydroformylation on rhodium zeolites X and Y I.Catalytic activity[J]. J Catal, 1985, 96(2): 563–573.
|
| [22] |
Hou C, Zhao G, Ji Y, et al. Hydroformylation of alkenes over rhodium supported on the metal-organic framework ZIF-8[J]. Nano Res, 2014, 7(9): 1364–1369.
DOI:10.1007/s12274-014-0501-4 |
| [23] |
Van Vu T, Kosslick H, Schulz A, et al. Hydroformylation of olefins over rhodium supported metal-organic framework catalysts of different structure[J]. Micropor Mesopor Mater, 2013, 177: 135–142.
DOI:10.1016/j.micromeso.2013.02.035 |
| [24] |
Vu T V, Kosslick H, Schulz A, et al. Influence of the textural properties of Rh/MOF-5 on the catalytic properties in the hydroformylation of olefins[J]. Micropor Mesopor Mater, 2012, 154: 100–106.
DOI:10.1016/j.micromeso.2011.11.052 |
| [25] |
Van Vu T, Kosslick H, Schulz A, et al. Selective hydroformylation of olefins over the rhodium supported large porous metal-organic framework MIL-101[J]. Appl Catal A-Gen, 2013, 468: 410–417.
DOI:10.1016/j.apcata.2013.09.011 |
| [26] |
Kainulainen T A, Niemelä M K, Krause A O I. Rh/C catalysts in ethene hydroformylation:The effect of different supports and pretreatments[J]. J Mol Catal A:Chem, 1999, 140(2): 173–184.
DOI:10.1016/S1381-1169(98)00223-4 |
| [27] |
Wang T, Wang W, Lyu Y, et al. Porous Rh/BINAP polymers as efficient heterogeneous catalysts for asymmetric hydroformylation of styrene:Enhanced enantioselectivity realized by flexible chiral nanopockets[J]. Chin J Catal, 2017, 38(4): 691–698.
DOI:10.1016/S1872-2067(17)62790-6 |
| [28] |
Nowotny M, Maschmeyer T, Johnson B F G, et al. Heterogeneous dinuclear Rhodium(ii) hydroformylation catalysts-performance evaluation and silsesquioxane-based chemical modeling[J]. Angew Chem Int Ed, 2001, 40(5): 955–958.
DOI:10.1002/1521-3773(20010302)40:5<955::AID-ANIE955>3.0.CO;2-G |
| [29] |
Li C, Sun K, Wang W, et al. Xantphos doped Rh/POPs-PPh3 catalyst for highly selective long-chain olefins hydroformylation:Chemical and DFT insights into Rh location and the roles of xantphos and PPh3[J]. J Catal, 2017, 353: 123–132.
DOI:10.1016/j.jcat.2017.07.022 |
| [30] |
Bourque S C, Maltais F, Xiao W J, et al. Hydroformylation reactions with rhodium-complexed dendrimers on silica[J]. J Am Chem Soc, 1999, 121(13): 3035–3038.
DOI:10.1021/ja983764b |
| [31] |
Arya P, Panda G, Rao N V, et al. Solid-phase catalysis:A biomimetic approach toward ligands on dendritic arms to explore recyclable hydroformylation reactions[J]. J Am Chem Soc, 2001, 123(12): 2889–2890.
DOI:10.1021/ja003854s |
| [32] |
Adint T T, Landis C R. Immobilized bisdiazaphospholane catalysts for asymmetric hydroformylation[J]. J Am Chem Soc, 2014, 136(22): 7943–7953.
DOI:10.1021/ja501568k |
| [33] |
Sakai N, Mano S, Nozaki K, et al. Highly enantioselective hydroformylation of olefins catalyzed by new phosphinephosphite-Rh(I) complexes[J]. J Am Chem Soc, 1993, 115(15): 7033–7034.
DOI:10.1021/ja00068a095 |
| [34] |
Jiang M, Yan L, Ding Y J, et al. Ultrastable 3V-PPh3 polymers supported single Rh sites for fixed-bed hydroformylation of olefins[J]. J Mol Catal A:Chem, 2015, 404/405: 211–217.
DOI:10.1016/j.molcata.2015.05.008 |
| [35] |
Wu Y, Wang T, Wang H, et al. Active catalyst construction for CO2 recycling via catalytic synthesis of N-doped carbon on supported Cu[J]. Nat Commun, 2019, 10(1): 2599.
|
| [36] |
Liu S, Dai X, Wang H, et al. Organic ligand-free hydroformylation with Rh particles as catalyst[J]. Chin J Chem, 2019, 38(2): 139–143.
|
| [37] |
Liu S, Dai X, Wang H, et al. Organic ligand and solvent free oxidative carbonylation of amine over Pd/TiO2 with unprecedented activity[J]. Green Chem, 2019, 21(15): 4040–4045.
DOI:10.1039/C9GC01577A |
| [38] |
Dai X, Adomeit S, Rabeah J, et al. Sustainable Co-synthesis of glycolic acid, formamides and formates from 1, 3-dihydroxyacetone by a Cu/Al2O3 catalyst with a single active sites[J]. Angew Chem Int Ed, 2019, 58(16): 5251–5255.
DOI:10.1002/anie.201814050 |
| [39] |
Liu S, Wang H, Dai X, et al. Organic ligand-free carbonylation reactions with unsupported bulk Pd as catalyst[J]. Green Chem, 2018, 20(15): 3457–3462.
DOI:10.1039/C8GC00740C |
| [40] |
Cui X, Dai X, Zhang Y, et al. Methylation of amines, nitrobenzenes and aromatic nitriles with carbon dioxide and molecular hydrogen[J]. Chem Sci, 2014, 5(2): 649–655.
DOI:10.1039/C3SC52676C |
| [41] |
Zhang Y, Dai X, Wang H, et al. Catalytic synthesis of formamides with carbon dioxide and amines[J]. Acta Phys -Chim Sin, 2018, 34(8): 845–857.
DOI:10.3866/PKU.WHXB201701081 |
| [42] |
Dai X, Wang B, Wang A, et al. Amine formylation with CO2 and H2 catalyzed by heterogeneous Pd/PAL catalyst[J]. Chin J Catal, 2019, 40(8): 1141–1146.
DOI:10.1016/S1872-2067(19)63397-8 |
| [43] |
Goertzen S L, Theriault K D, Oickle A M, et al. Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination[J]. Carbon, 2010, 48(4): 1252–1261.
DOI:10.1016/j.carbon.2009.11.050 |
| [44] |
Chaudhari R V, Bhanage B M, Deshpande R M, et al. Enhancement of interfacial catalysis in a biphasic system using catalyst-binding ligands[J]. Nature, 1995, 373(6514): 501–503.
DOI:10.1038/373501a0 |
| [45] |
Sun Q, Dai Z, Liu X, et al. Highly efficient heterogeneous hydroformylation over Rh-metalated porous organic polymers:Synergistic effect of high ligand concentration and flexible framework[J]. J Am Chem Soc, 2015, 137(15): 5204–5209.
DOI:10.1021/jacs.5b02122 |
| [46] |
Diao Y, Li J, Wang L, et al. Ethylene hydroformylation in imidazolium-based ionic liquids catalyzed by rhodium-phosphine complexes[J]. Catal Today, 2013, 200: 54–62.
DOI:10.1016/j.cattod.2012.06.031 |
| [47] |
Dossi C, Fusi A, Garlaschelli L, et al. Ethylene hydroformylation with the silica-supported K2Rh12(CO)30 cluster:Evidence for vapor-phase cluster catalysis[J]. Catal Lett, 1991, 11(3/6): 335–339.
|
 2020, Vol. 34
2020, Vol. 34