2. 中国科学院大学, 北京 100049
2. University of Chinese Academy of Sciences, Beijing 100049, China
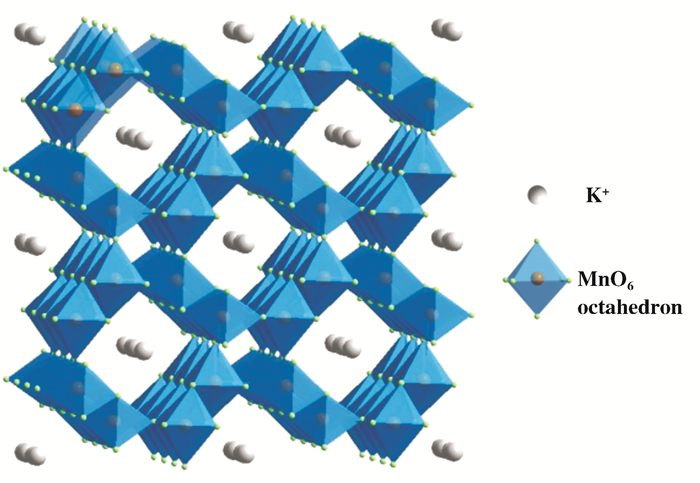
氧化锰八面体分子筛(Manganese oxide octahedral molecular sieves, OMS-2)是一种类似于沸石分子筛结构的新型材料, 由2×2的八面体结构单元MnO6以边角共享方式组成的孔径为0.46 nm的一维孔道结构, 孔道内含有K+以平衡整体价态和支撑孔道结构(图 1)[1].自1994年, Suib团队[2]首次使用水热法合成出具有隐钾锰矿晶相(cryptomelane)的OMS-2, 由于在氧化、吸附、离子交换和导电性等方面的特性, OMS-2被逐渐应用于环保、电极材料、半导体、分离和医疗等诸多领域[3]. OMS-2的理想晶体化学式为KMn8O16·nH2O, 具有Mn4+、Mn3+和Mn2+共存的混合价态、约3.8的平均氧化态, 丰富的表面缺陷空位、活泼的晶格氧、温和的酸碱性和易于离子改性的结构等特点, 近年来作为新型催化剂在有机分子合成和环境催化领域得到了广泛应用[4].
|
图 1 二氧化锰分子筛OMS-2结构示意图 Fig.1 Structure diagram of Manganese oxide octahedral molecular sieves, OMS-2 |
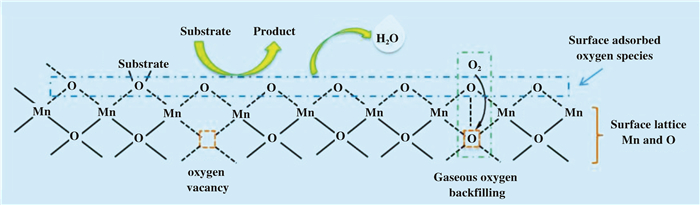
OMS-2有多种制备方法, 如:水热法[3]、回流法[5]、超声法[5]、固相法[6]、微波法[7]、以及通过离子液体或者高温焙烧诱导的晶相转变法[8-9]等.通过调节制备过程中的反应时间、温度、pH值和反应前驱体可控制OMS-2颗粒尺寸、形貌和表面缺陷数量, 从而使其表现出不同的催化性能(图 2).此外, 为了进一步提升OMS-2的催化性能, 还可以通过掺杂金属离子对OMS-2进行改性[2, 10-18], 由于金属离子半径大小不同, 可能掺杂到八面体分子筛骨架或孔道中, 使材料的酸碱度、孔隙率、晶格氧迁移性与吸附性等性质发生改变[19-23]. OMS-2能够释放出氧的数量大于早期合成的钡镁锰矿(OMS-1), 因此具有更强的催化氧化活性, 氧化反应机制符合Mars-van-Krevelen反应机理[24](图 3).底物分子中的X-H(X=C, N, O等)与催化剂表面吸附氧或氧空位相互作用, 吸附在催化剂表面, 通过C—H键断裂得到脱氢产物.反应过程中, 两个H原子和一个表面吸附O原子结合以H2O分子的形式离开反应界面, 催化剂表面得以产生氧空位, 反应气氛中的氧分子被活化填补氧空位, 从而补充催化剂中缺失的氧物种, 使催化剂骨架结构及催化活性得以保持.

|
图 2
不同形貌OMS-2材料制备
Fig.2
OMS-2 materials with different particle sizes were prepared by different preparation methods
(a)回流法; (b)固相法; (c)微波法; (d)超声法 (授权转载于美国化学学会) (a) reflux; (b) solvent free; (c) microwave-assisted reflux; (d) ultrasound-assisted synthesis (Copyright permission granted by the American Chemical Society) |
|
图 3 OMS-2催化氧化过程可能反应机理 Fig.3 The possible mechanism of catalytic oxidation over OMS-2 |
随着绿色化学的发展, 现代化工急需解决高能耗、重污染问题, 尤其是应用于有机化学品合成与转化的氧化反应, 亟待革新以往使用化学计量氧化剂(如高锰酸钾和4价铬试剂)的转化过程, 发展使用O2、H2O2等绿色氧化剂的催化氧化过程是现代化工中清洁合成发展的必然趋势. OMS-2催化剂能在温和条件下同O2、H2O2等氧化剂之间发生快速电子传递, 实现对绿色氧化剂的高效活化, 氧化反应完成后只产生唯一副产物H2O.而且, 由于其具有敞开的孔道结构、优异的氧化性和温和的酸碱性、可控的形貌尺寸和比表面积, OMS-2作为非均相催化剂在有机分子催化氧化反应中表现出良好的催化活性、反应选择性和结构稳定性[25-41].此外, OMS-2还可作为电子转移介质(electron transfer mediators, ETMs)与活性金属M的载体, 两者之间实现多步电子传递, 有效降低氧化还原反应能垒[42-43, 44-49].目前已有多篇中英文综述从OMS-2制备表征[1]、在生物质和CO转化[50]及环境污染物降解[51]方面的应用进行了介绍.我们综述了近年来OMS-2催化剂在有机分子清洁氧化合成的应用进展, 及其作为具有电子转移介质性能的功能化载体材料的研究进展.
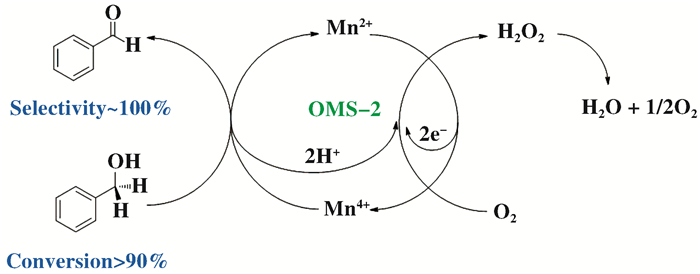
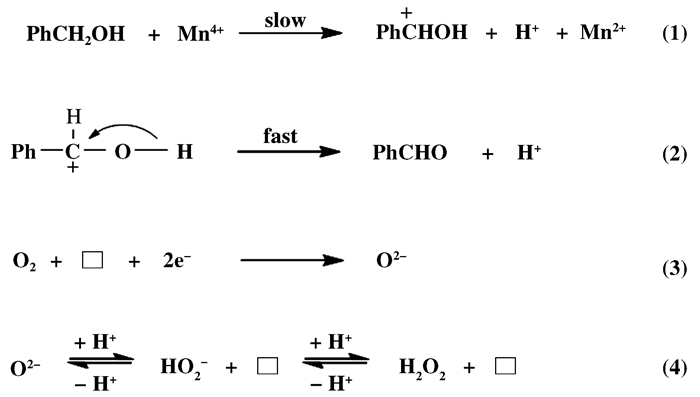
1 OMS-2在有机合成中的催化应用 1.1 醛类化合物的合成在制药和有机合成过程中, 醛是很有价值的化工中间体, 用一步法将醇类选择性氧化为相应的醛类化合物具有重要意义[52-55]. 2001年, 人们首次以OMS-2作为催化剂, 以空气为氧化剂, 将醇类化合物选择性氧化为相应的醛, 而无副产物羧酸生成[56]. Son等[56-57]使用同位素标记法(18O)对该反应进行机理研究, 发现反应符合Mars van Krevelen机理, 即在催化氧化过程中, 首先苯甲醇被氧化为缺电子中间体, 同时OMS-2中的Mn4+被还原为Mn2+(步骤1为速控步骤), 中间体快速生成苯甲醛(步骤2), 继而分子氧得到两电子被还原为晶格氧O2-(步骤3), 晶格氧与H+反应生成H2O2, 最后H2O2在OMS-2表面分解生成H2O(图 5).为进一步拓展底物适用范围, Mewada课题组[58]制备出负载Ag的OMS-2, 高选择性地将正辛醇氧化为正辛醛; Nie等[59]也将类似的OMS-2/O2催化体系应用于生物质平台化合物5-羟甲基糠醛选择性氧化2, 5-二甲酰基呋喃中, 得到了优异的收率.
|
图 4 OMS-2选择性催化苯甲醇与分子氧制备苯甲醛机理[57] Fig.4 The catalytic mechanism of benzyl alcohol oxidation with molecular oxygen and OMS-2[57] |
|
图 5 OMS-2选择性催化氧化苯甲醇生成苯甲醛反应路线和氧物种转变过程 Fig.5 The reaction route of selective catalytic oxidation of benzyl alcohol to benzaldehyde under OMS-2 and the transformation of oxygen species |
Chen课题组[60]在回流条件下, 将碱金属/碱土金属M(Li+, Na+, Mg2+, Ca2+, Sr2+, Zn2+, Al3+, La3+)与OMS-2孔道内的K+进行离子交换, 发现Na+改性的OMS-2在乙醇氧化为乙醛的气相反应中表现出优异的催化氧化性能.通过动力学研究, 研究人员提出可能的反应机理:催化剂表面氧空位活化O2形成超氧自由基O2-; 继而O2-活化乙醇中O—H键形成乙醇盐; 晶格氧或O2-吸附乙醇中的α-C-H, 生成乙醛和还原态锰离子; 氧化过程形成的H2O和O2在催化剂表面脱附, 释放氧空位, 从而完成催化循环.在此过程中, 催化剂表面氧空位能够增强乙醇在催化剂表面的吸附, 而Na+掺杂提高了催化剂表面的碱性和氧空位, 从而表现出优异的催化性能.
此外, 研究发现OMS-2催化剂的形貌对其活性具有重要影响. Yin课题组[61]采用固相法、回流法和水热法分别合成出棒状、纤维状、树枝状和鸟巢状等不同形貌的OMS-2催化剂(图 6), 并将其应用于对氯甲苯选择性氧化合成对氯苯甲醛的反应.他们发现:鸟巢状OMS-2含有更高比例的Mn3+、丰富的表面氧空位、活泼的晶格氧与较强的氧化还原性能, 能够有效活化氧分子向氧空位迁移补充晶格氧, 从而表现出最佳的催化氧化活性.

|
图 6
不同形貌OMS-2的SEM图像
Fig.6
SEM images of (a) rod-like; (b) fibrous-like; (c) dendritic-like; and (d)nest-like OMS-2[61]
(a)棒状; (b)纤维状; (c)树枝状; (d)鸟巢状[61] (授权转载于文献[61], 催化通讯杂志版权2014) (Reprinted with permission from references[61], Copyright 2014, Catalysis Communications) |
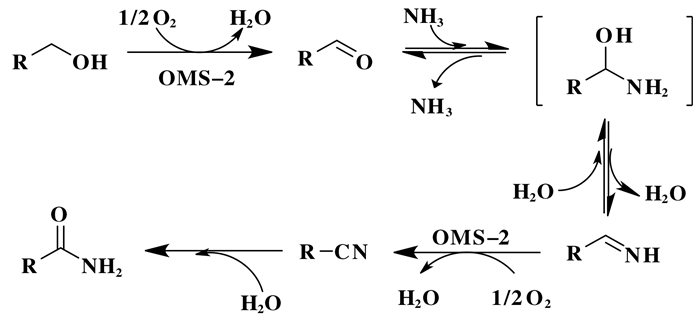
作为一类应用广泛的有机骨架, 酰胺的合成工艺开发是有机合成中重要的研究课题, 特别是一级醇同氨水的氧化酰胺化反应, 是酰胺清洁合成的重要途径之一. Yamaguchi等[62]以OMS-2作为催化剂, 在0.3 MPa的O2下, 实现了一级醇同氨水之间的氧化酰胺化反应, 合成了含有广泛官能团的酰胺化合物, OMS-2在反应中表现出优异的催化选择性和结构稳定性, 能够有效循环使用多次, 无金属析出.机理研究表明, 由醇氧化得到的醛同氨水通过半胺醛中间体得到醛亚胺后, OMS-2能够选择性催化氧化脱氢生成目标中间体腈类化合物, 而不容易发生氧化脱水反应, 从而提高了反应选择性, 得到目标酰胺类化合物(图 7).
|
图 7 OMS-2催化伯醇与氨反应生成酰胺可能反应路径[62] Fig.7 Possible reaction pathway for OMS-2-catalyzed transformation of primary alcohols to primary amides[62] |
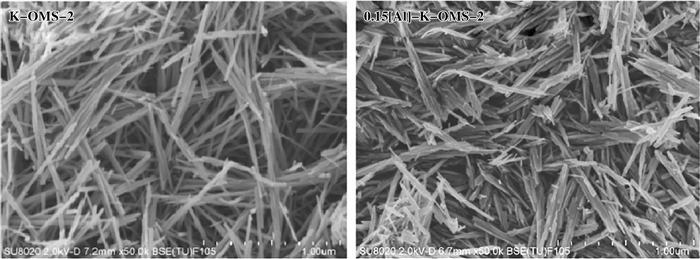
鉴于化石资源储量有限及其引发的环境问题, 生物质催化转化吸引了越来越多研究者的兴趣[63].例如, 平台化合物5-羟甲基糠醛(HMF)的酰胺化为可再生资源清洁合成含氮化合物提供了新途径, OMS-2催化材料在此领域也取得了许多进展[63-71]. Xu研究团队[65]利用OMS-2易于离子交换的结构特性, 制备出多种金属掺杂改性的OMS-2催化剂, 用于O2气氛下催化HMF同氨水的酰胺化反应, 高选择性地得到了2, 5-呋喃二甲酰胺化合物(图 8).他们发现, 掺杂金属种类(In3+、Cr3+、Co2+、Ni2+、Cu2+、Zn2+、Al3+)在优化催化剂性能方面起着关键作用, 其中Al3+与Mnn+的离子半径相似, 可通过离子交换进入OMS-2骨架结构, Al3+的掺杂显著增强了OMS-2晶格氧反应活性与表面酸性, 表面酸性提高有利于底物NH3吸附, 从而提高底物的转化率, 铝离子掺杂的OMS-2材料氧化活性位和酸性位的共存对反应至关重要.
|
图 8
SEM图像[65]
Fig.8
SEM images of K-OMS-2 and 0.15[Al]-K-OMS-2[65]
(a) K-OMS-2; (b) 0.15[Al]-K-OMS-2 (授权转载于文献[65], 美国化学会可持续化学与工程杂志版权2018) (Reprinted with permission from references[65], Copyright 2018, ACSSUSTAINABLE CHEMISTRY & ENGINEERING) |
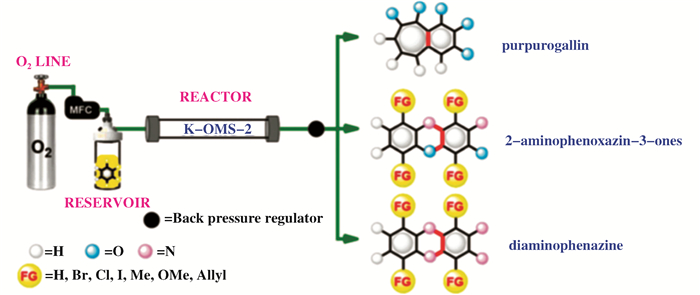
氮杂环化合物作为功能化的有机分子骨架, 广泛应用于药物合成、有机材料和传感器件等领域, 其合成过程也要求更加绿色, 更具原子经济性和选择性.最近, Ferlin等[72]使用H2O2和O2作为混合氧化剂, 在OMS-2催化活化下, 有效模拟过氧化物酶氧化活化邻氨基苯酚、邻苯二胺和焦棓酚的C—H键, 通过分子间的氧化偶联, 在室温下选择性合成多种药物结构分子, 如2-氨基苯并恶嗪酮、红棓酚及其衍生物(图 9).他们发现, 相比传统的辣根过氧化物酶催化剂, 金属氧化物OMS-2对温度、溶剂及底物的适用性更为广泛, 并且结构稳定性好, 可重复使用多次, 无金属离子脱落.他们推测OMS-2含有K+的独特三维孔道结构是其具有优异催化氧化活性与稳定性的关键因素.这一研究, 显示了OMS-2催化剂可以作为生物酶催化剂的有效模拟物, 并用于高效合成多种药物结构分子的应用中, 为今后合成无机催化剂模拟生物酶催化提供了依据.

|
图 9 OMS-2催化氧化合成2-氨基苯并恶嗪酮与红棓酚衍生物[72] Fig.9 OMS-2-catalyzed 2-aminophenol to 2-aminophenoxazin-3-one and purpurogallin[72] |
Ferlin等[73-74]将OMS-2催化剂与连续流动化学技术结合, 针对2-氨基苯并恶嗪酮和红棓酚等药物活性分子的合成, 发展了高效且废物最小化的连续流动合成工艺(图 10).他们以O2为终端氧化剂, 通过OMS-2催化氧化邻氨基苯酚、邻苯二胺和焦棓酚的分子间偶联反应, 在连续流动条件下合成目标产物, 收率高达99%.该催化体系以低毒性、可循环使用的环戊基甲醚为绿色溶剂, 使得反应体系更加经济安全, O2的参与促进了底物分子的氧化反应过程, 同时加速了催化剂中还原态Mn离子的氧化, 保证催化体系稳定高效进行.该研究中, OMS-2催化剂首次同连续流动化学技术结合使用, 可通过适当调整O2压力与物料停留时间提高反应效率, 有效解决了长期以来OMS-2催化中TON和TOF过低的问题.
|
图 10 连续流动反应工艺[73] Fig.10 Flow reactor scheme[73] (授权转载于文献[73], 绿色化学版权2020) (Reprinted with permission from references[73], Copyright 2020, Green Chemistry) |
含氮杂环的氧化脱氢是杂环化合物合成的重要途径之一, 基于OMS-2的改性催化剂也被应用于该方面研究[38, 42-43, 75-84].最近, 赵培庆课题组[38]利用磷钨酸钠作为改性试剂, 制备出具有混合晶相的OMS-2纳米复合催化剂, 并应用于N-杂环化合物氧化脱氢反应中; 他们发现, 掺杂2%(摩尔分数)的改性试剂后, 复合催化剂的比表面积(204 m2/g)和晶格氧含量(80.4%)显著提高, 可高选择性的得到喹啉、喹唑啉、咔啉和吲哚等结构的杂环化合物, 且改性后的OMS-2复合催化剂结构稳定, 可重复循环使用多次(图 11).这项研究打破了单一隐钾锰矿晶相OMS-2比混合晶相具有更高催化活性的普遍认知, 通过材料混合晶相和优势生长晶面的精准控制, 能够产生多种吸附活性位, 促进混合晶相间快速电子传递, 有效降低反应活化能, 提高催化效率.
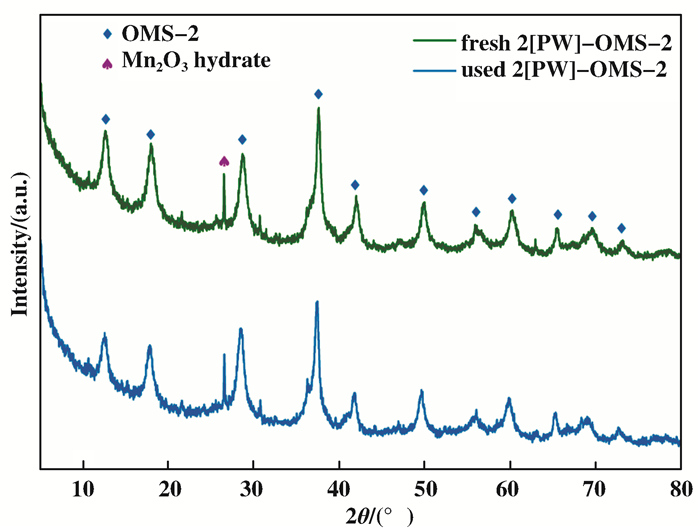
|
图 11 新制与使用10次后2[PW]-OMS-2的XRD谱图[38] Fig.11 The XRD patterns of fresh 2[PW]-OMS-2 and 2[PW]-OMS-2 reuse for 10 times[38] |
另外, 赵培庆等人近年来拓展了一系列OMS-2催化的含N杂环化合物清洁合成路线(图 12).如:肉桂醇和苯甲脒选择性合成嘧啶类化合物[78]; 苄醇和苯甲脒催化合成1, 3, 5-三嗪类化合物[79]; 醇类和芳基脒类化合物催化合成喹唑啉类化合物[80]等.以上非均相催化体系中无需使用碱和配体, 底物适用范围广, 使用O2为氧化剂, 且OMS-2催化剂表现出良好的催化选择性和结构稳定性.这些结果显示, 通过催化反应体系的构筑, OMS-2催化剂能够促进有机分子间选择性的氧化反应, 实现目标有机分子骨架高效精准合成.

|
图 12 OMS-2催化醇与脒类合成含N-杂环化合物 Fig.12 Synthesis of N-containing heterocycles from alcohols and amidines catalyzed by OMS-2 |
迄今为止, 已开发出许多以O2作为氧化剂的OMS-2催化氧化体系, 但H2O2参与的清洁合成体系鲜有报道, 主要原因为H2O2试剂中含水量大, 在在有机反应体系中难以兼容.赵培庆等人提出了以OMS-2为催化剂, H2O2为终端氧化剂, 在室温下氧化合成苯并恶唑和苯并噻唑的反应路径[81].该催化体系反应条件温和, 以碳酸二甲酯作为绿色溶剂, 实现了苯并恶唑类化合物的绿色清洁合成.更重要的是, OMS-2催化剂在克级规模反应中可多次重复使用, 使得该催化体系与以往的体系相比更具实用性与工业应用潜力.
1.4 偶氮苯类化合物的合成偶氮苯及其衍生物是一类重要化合物, 被广泛应用于食品添加剂、药物中间体、染料等方面. Wang课题组[85]利用OMS-2作为非均相催化剂、O2作为终端氧化剂催化苯胺氧化偶联实现清洁合成偶氮苯的反应路线.该催化体系不但可以实现苯胺自偶联, 在不对称偶氮苯的合成中也表现出良好的转化率与选择性(图 13).基于EPR和XRD表征分析, 他们认为OMS-2中的氧空位在促进电子转移和激活分子氧方面起着关键作用, 并且提出了单电子转移(SET)反应机理:苯胺N原子上的孤对电子与OMS-2表面氧空位互相作用, 从而吸附在催化剂表面氧空位, 电子由苯胺N原子向氧空位进行单电子转移, 形成苯胺自由基阳离子, 进而活化Mn—O键将Mn4+还原为Mn3+/Mn2+, 苯胺自由基阳离子经过进一步电子转移脱氢形成偶氮苯, 还原态OMS-2被O2重新氧化进入下一个反应循环.其中, 混合价态的Mn离子在促进电子转移和活化氧气方面起到了关键作用, 低价态Mn离子含量越高, 对应的氧空位越多, 进而有利于苯胺分子的吸附、促进O2分子活化, 由此可见, 不饱和配位的Mn—O键对该催化过程至关重要.
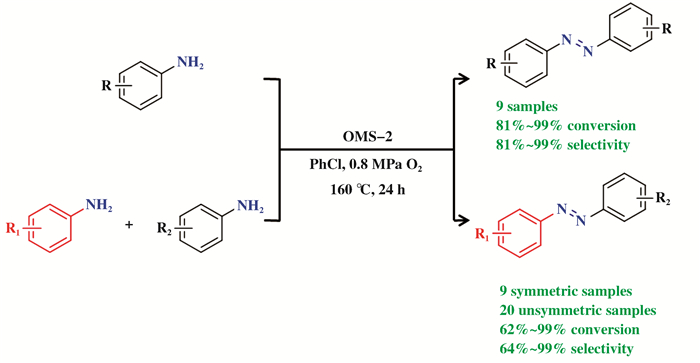
|
图 13 OMS-2催化的苯胺氧化偶联合成偶氮苯类化合物[85] Fig.13 OMS-2-catalyzed oxidative coupling of anilines to azobenzenes[85] |
从环境友好角度来说, 清洁催化氧化过程要求以O2或H2O2作为氧化剂, 但是直接利用O2或H2O2作为有效氧化剂需要克服较高的氧化还原能垒, 在动力学上是不利的.因此, 研究人员开发出含有电子转移介质的多步催化氧化反应体系[44-45], 将具有催化活性的金属M负载在具有ETM性质的载体材料上, ETM通过单电子转移来氧化还原态的活性金属M, 而O2/H2O2将还原态的ETM再氧化成高价态, 通过多步的快速电子传递来完成催化氧化反应, 直接通过负载型催化剂M/ETM内部的两组分相互作用来降低氧化还原反应能垒(图 14).
Mizuno课题组[46]发现在氧化还原势能上CuO/Cu2O=0.67 V, MnO2/Mn2O3=1.04 V, O2/H2O=1.23 V, 直接用O2氧化低价态Cu非常困难, 但O2氧化低价态Mn较容易, 类似的, 高价态Mn氧化低价态Cu较容易, 因此OMS-2可在Cu与O2之间作为ETM, 整个反应中Cu2+作为反应底物的催化剂, O2作为终端氧化剂, 氧化反应便可以在负载型Cu/OMS-2催化下, 沿着一条低能垒路径完成.他们在Cu(II)的溶液中, 以OMS-2作为具有电子转移功能的催化剂载体, 通过共沉淀法制备了Cu(OH)x/OMS-2催化材料, 成功应用于末端炔烃的氧化偶联中(图 15), 催化体系能够有效降低直接氧化的反应能垒, 具有反应条件温和, 催化剂用量少, 无碱添加, 底物适用性广和可循环使用的特点.
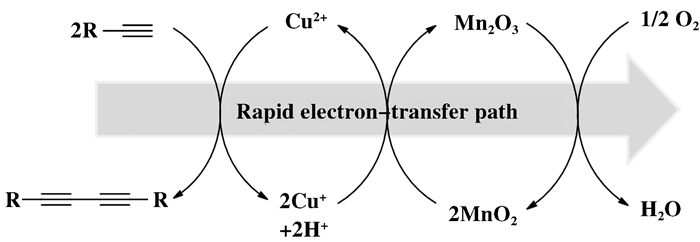
|
图 15 Cu(OH)x/OMS-2催化低能垒路径下的末端炔烃氧化偶联反应[46] Fig.15 Cu(OH)x/OMS-2-catalyzed homo-coupling of alkynes under low-energy pathway[46] (转载于文献[46], 美国化学会催化杂志版权2011) (Reprinted with permission from references[46], Copyright 2011, ACS Catalysis) |
Mizuno课题组[47]制备了Au/OMS-2催化剂, 首次实现在非均相催化体系中饱和酮的α, β-脱氢反应(图 16).该体系中, OMS-2作为催化活性金属的载体与ETM, 使Au纳米粒子与O2之间发生间接快速电子转移, 显著降低了反应能垒.相比于传统合成方法, Au/OMS-2催化体系反应条件温和, 能够有效活化Air/O2作为终端氧化剂, 拓展了底物适用范围, 避免了化学计量氢受体的添加.他们还开发出Au/OMS-2作为非均相催化剂的α, β-不饱和醛的氧化脱氢[48]、末端炔烃与单氢硅烷氧化偶联反应[49].
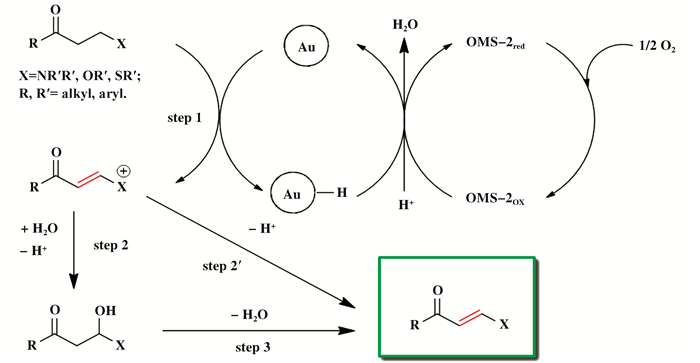
|
图 16 Au/OMS-2催化氧化饱和酮经过α, β-脱氢得到α, β-不饱和酮[47] Fig.16 Possible reaction mechanism for the Au/OMS-2-catalyzed oxidative α, β-dehydrogenation ofβ-heteroatom-substituted saturatedketones[47] (授权转载于文献[47], 化学通讯版权2016) (Reprinted with permission from references[47], Copyright 2016, ACS Chemical Communications) |
同时, 赵培庆等人也合成了一系列OMS-2负载型催化材料, 如: CuOx/OMS-2和CuOx/H-OMS-2, 这些催化剂均以Cu为催化活性金属, OMS-2作为ETM和载体, 在二者构成的低能垒快速电子传递路径下, 以空气或O2为有效氧化剂, 实现了咪唑并吡啶类(图 17)、苯并恶唑类、三嗪类、喹唑啉酮类等杂环化合物的清洁合成[42-43, 82-84].以上催化剂的使用, 能够降低催化活性金属的用量、避免了酸碱和配体的使用、在温和的反应条件下提高了反应选择性, 催化剂具有良好的结构稳定性, 可重复使用多次而无活性金属脱落.
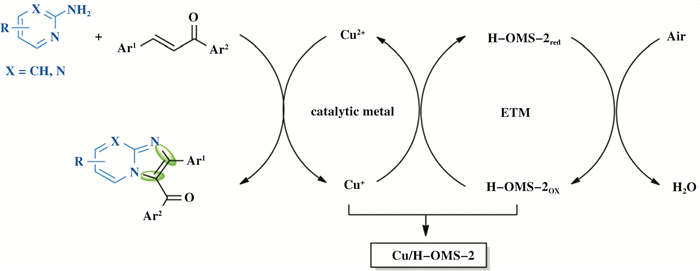
|
图 17 以酸改性OMS-2为ETM, 铜催化多步氧化反应合成咪唑并吡啶和咪唑并嘧啶[43] Fig.17 Cu-catalyzed multi-step oxidations using acid-modified OMS-2 as an ETM for the synthesis of imidazo[1, 2-a]pyridines and imidazo[1, 2-a]pyrimidines[43] (授权转载于文献[43], 催化科学与技术版权2016) (Reprinted with permission from references[43], Copyright 2016, Catalysis Science & Technology) |
氧化锰八面体分子筛因其混合价态锰离子、优异的氧化性和丰富的表面缺陷空位、活泼的晶格氧和易于离子改性的结构特点, 在多种有机分子和精细化学品的清洁合成反应中表现出高效的催化性能.然而, 目前人们对OMS-2催化材料的研究处于初期阶段, 许多科学问题尚未得到解决, 如一些反应过程机制不明确, 对催化剂中不同金属组分之间相互作用机制缺乏理解, OMS-2难以实现千克级大规模制备, 在复杂分子或多组分反应合成中催化效果不佳, 反应TON/TOF较低等.因此, 今后的研究工作需要紧密结合理论模拟和原位过程研究, 深入理解OMS-2参与的催化过程反应机理, 开发OMS-2的绿色创新制备路线, 争取实现OMS-2催化材料活性位点的精准构筑与调控.
| [1] |
Suib S. Porous manganese oxide octahedral molecular sieves and octahedral layered materials[J]. Acc Chem Res, 2007, 41(4): 479–487.
|
| [2] |
DeGuzman R, Shen Y, Neth E, et al. Synthesis and characterization of octahedral molecular sieves (OMS-2) having the hollandite structure[J]. Chem Mater, 1994, 6(6): 815–821.
|
| [3] |
Zhang Yi(张溢), Shi Jin(石琎), Zhang Yi-xiang(张志翔), et al. Study on simultaneous removal of formaldehyde and ozone by manganese oxygen octahedral molecular sieve at room temperature(锰氧八面体分子筛室温同时去除甲醛和臭氧的性能研究)[J]. J Mol Catal (China)(分子催化), 2019, 33(3): 201–208.
|
| [4] |
Tao F. Metal nanoparticles for catalysis: Advances and applications[M]. England: RSC Press, 2014: 235-246.
|
| [5] |
Dharmarathna S, King'ondu C, Pedrick W, et al. Direct sonochemical synthesis of manganese octahedral molecular sieve (OMS-2) nanomaterials using cosolvent systems, their characterization, and catalytic applications[J]. Chem Mater, 2012, 24(4): 705–712.
|
| [6] |
Ding Y, Shen X, Sithambaram S, et al. Synthesis and catalytic activity of cryptomelane-type manganese dioxide nanomaterials produced by a novel solvent-free method[J]. Chem Mater, 2005, 17(21): 5382–5389.
|
| [7] |
Nyutu E, Chen C, Sithambaram S, et al. Systematic control of particle size in rapid open-vessel microwave synthesis of K-OMS-2 nanofibers[J]. J Phys Chem C,, 2008, 112(17): 6786–6793.
|
| [8] |
Yan G, Lian Y, Gu Y, et al. Phase and morphology transformation of MnO2 induced by ionic liquids toward efficient water oxidation[J]. ACS Catal, 2018, 8(11): 10137–10147.
|
| [9] |
Subramanian N, Viswanathan B, Varadarajan T. A facile, morphology-controlled synthesis of potassium-containing manganese oxide nanostructures for electrochemical supercapacitor application[J]. RSC Adv, 2014, 4(64): 33911–33922.
|
| [10] |
Cai J, Liu J, Willis W, et al. Framework doping of iron in tunnel structure cryptomelane[J]. Chem Mater, 2001, 13(7): 2413–2422.
|
| [11] |
Calvert C, Joesten R, Ngala K, et al. Synthesis, characterization, and rietveld refinement of tungsten-framework-doped porous manganese oxide (K-OMS-2) material[J]. Chem Mater, 2008, 20(20): 6382–6388.
|
| [12] |
Chen X, Shen Y, Suib S, et al. Catalytic decomposition of 2-propanol over different metal-cation-doped OMS-2 materials[J]. J Catal, 2001, 197(2): 292–302.
|
| [13] |
Hashem A, Mohamed H, Bahloul A, et al. Thermal stabilization of tin- and cobalt-doped manganese dioxide[J]. Ionics, 2008, 14(1): 7–14.
|
| [14] |
Jothiramalingam R, Viswanathan B, Varadarajan T. Synthesis and structural characterization of copper incorporated manganese oxide OMS-2 materials synthesized via potassium birnessite[J]. Mater Chem Phys, 2006, 100(2): 257–261.
|
| [15] |
Jothiramalingam R, Viswanathan B, Varadarajan T. Synthesis, characterization and catalytic oxidation activity of zirconium doped K-OMS-2 type manganese oxide materials[J]. J Mol Catal A: Chem, 2006, 252(1): 49–55.
|
| [16] |
Kingo'ndu C, Opembe N, Chen C, et al. Manganese oxide octahedral molecular sieves (OMS-2) multiple framework substitutions: A new route to OMS-2 particle size and morphology control[J]. Adv Funct Mater, 2011, 21(2): 312–323.
|
| [17] |
Onda A, Hara A, Kajiyoshi K, et al. Synthesis of manganese oxide octahedral molecular sieves containing cobalt, nickel, or magnesium, and the catalytic properties for hydration of acrylonitrile[J]. Appl Catal A, 2007, 321(1): 71–78.
|
| [18] |
Wang R, Li J. OMS-2 catalysts for formaldehyde oxidation: Effects of Ce and Pt on structure and performance of the catalysts[J]. Catal Lett, 2009, 131: 500–505.
|
| [19] |
Iyer A, Galindo H, Sithambaram S, et al. Nanoscale manganese oxide octahedral molecular sieves (OMS-2) as efficient photocatalysts in 2-propanol oxidation[J]. Appl Catal A, 2010, 375(2): 295–302.
|
| [20] |
Kumar R, Sithambaram S, Suib S. Cyclohexane oxidation catalyzed by manganese oxide octahedral molecular sieves-effect of acidity of the catalyst[J]. J Catal, 2009, 262(2): 304–313.
|
| [21] |
Sithambaram S, Xu L, Chen C, et al. Manganese octahedral molecular sieve catalysts for selective styrene oxide ring opening[J]. Catal Today, 2009, 140(4): 162–168.
|
| [22] |
Sriskandakumar T, Opembe N, Chen C, et al. Green decomposition of organic dyes using octahedral molecular sieve manganese oxide catalysts[J]. J Phys Chem A, 2009, 113(8): 1523–1530.
|
| [23] |
Huang H, Chen C, Xu L, et al. Single-step synthesis of manganese oxide octahedral molecular sieves with large pore sizes[J]. Chem Commun, 2010, 46(32): 5945–5947.
|
| [24] |
Makwana V, Son Y, Howell A, et al. The role of lattice oxygen in selective benzyl alcohol oxidation using OMS-2 catalyst: A kinetic and isotope-labeling study[J]. J Catal, 2002, 210(1): 46–52.
|
| [25] |
Najafpour M, Holynska M, Salimi S. Applications of the "nano to bulk" Mn oxides: Mn oxide as a swiss army knife[J]. Coor Chem Rev, 2015, 285: 65–75.
|
| [26] |
Makwana V, Son Y, Howell A, et al. The role of lattice oxygen in selective benzyl alcohol oxidation using OMS-2 catalyst: A kinetic and isotope-labeling study[J]. J Catal, 2002, 210(1): 46–52.
|
| [27] |
Liu J, Makwana V, Cai J, et al. Effects of alkali metal and ammonium cation templates on nanofibrous cryptomelane-type manganese oxide octahedral molecular sieves (OMS-2)[J]. J Phys Chem B, 2003, 107(35): 9185–9194.
|
| [28] |
Makwana V, Garces L, Liu J, et al. Selective oxidation of alcohols using octahedral molecular sieves: Influence of synthesis method and property-activity relations[J]. Catal Today, 2003, 85(4): 225–233.
|
| [29] |
Dharmarathna S, King'ondu C, Pedrick W, et al. Direct sonochemical synthesis of manganese octahedral molecular sieve (OMS-2) nanomaterials using cosolvent systems, their characterization, and catalytic applications[J]. Chem Mater, 2012, 24(4): 705–712.
|
| [30] |
Opembe N, King'Ondu C, Suib S. Efficient oxidation of 2, 3, 6-trimethyl phenol using non-exchanged and H+ exchanged manganese oxide octahedral molecular sieves (K-OMS-2 and H-K-OMS-2) as catalysts[J]. Catal Lett, 2012, 142: 427–432.
|
| [31] |
Zhou H, Wang J, Chen X, et al. Studies of oxidative dehydrogenation of ethanol over manganese oxide octahedral molecular sieve catalysts[J]. Microporous Mesoporous Mater, 1998, 21(6): 315–324.
|
| [32] |
Krishnan V, Suib S. Oxidative dehydrogenation of 1-butene over manganese oxide octahedral molecular sieves[J]. J Catal, 1999, 184(2): 305–315.
|
| [33] |
Nicolas-Tolentino E, Tian Z, Zhou H, et al. Effects of Cu2+ ions on the structure and reactivity of todorokite- and cryptomelane-type manganese oxide octahedral molecular sieves[J]. Chem Mater, 1999, 11(7): 1733–1741.
|
| [34] |
Ghosh R, Shen X, Villegas J, et al. Role of manganese oxide octahedral molecular sieves in styrene epoxidation[J]. J Phys Chem B, 2006, 110(14): 7592–7599.
|
| [35] |
Sithambaram S, Xu L, Chen C, et al. Manganese octahedral molecular sieve catalysts for selective styrene oxide ring opening[J]. Catal Today, 2009, 140(4): 162–168.
|
| [36] |
Zhang zhen-zhen(张珍珍), Li Xin-heng(李鑫恒). Recent advances on catalytic oxidation of formaldehyde(基于催化氧化技术去除甲醛的研究进展)[J]. J Mol Catal (China)(分子催化), 2019, 33(4): 382–390.
|
| [37] |
Kumar R, Garces L, Son Y, et al. Manganese oxide octahedral molecular sieve catalysts for synthesis of 2-aminodiphenylamine[J]. J Catal, 2005, 236(2): 387–391.
|
| [38] |
Bi X, Tang T, Meng X, et al. Aerobic oxidative dehydrogenation of N-heterocycles over OMS-2-basednanocomposite catalysts: Preparation, characterization and kinetic study[J]. Catal Sci Technol, 2020, 10(2): 360–371.
|
| [39] |
Kumar R, Sithambaram S, Suib S. Cyclohexane oxidation catalyzed by manganese oxide octahedralmolecular sieves effect of acidity of the catalyst[J]. J Catal, 2009, 262(2): 304–313.
|
| [40] |
Iyer A, Del-Pilar J, King'Ondu C, et al. Water oxidation catalysis using amorphous manganese oxides, octahedral molecular sieves (OMS-2), and octahedral layered (OL-1) manganese oxide structures[J]. J Phys Chem C, 2012, 116(10): 6474–6483.
|
| [41] |
Yamaguchi K, Kobayashi H, Oishi T, et al. Heterogeneously catalyzed synthesis of primary amides directly from primary alcohols and aqueous ammonia[J]. Angew Chem, 2012, 124(2): 559–562.
|
| [42] |
Meng X, Yu C, Chen G, et al. Heterogeneous biomimetic aerobic synthesis of 3-iodoimidazo[1, 2-a]pyridines via CuOx/OMS-2-catalyzed tandem cyclization/iodination and their late-stage functionalization[J]. Catal Sci Technol, 2015, 5(1): 372–379.
|
| [43] |
Meng X, Zhang J, Chen B, et al. Copper supported on H+-modified manganese oxide octahedral molecular sieves (Cu/H-OMS-2) as a heterogeneous biomimetic catalyst for the synthesis of imidazo[1, 2-a]-N-heterocycles[J]. Catal Sci Technol, 2016, 6(3): 890–896.
|
| [44] |
Piera J, Bäckvall J. Catalytic oxidation of organic substrates by molecular oxygen and hydrogen peroxide by multistep electron transfer-A biomimetic approach[J]. Angew Chem Int Ed, 2008, 47(19): 3506–3516.
|
| [45] |
Babu B, Meng X, Bäckvall . Aerobic oxidative coupling of arenes and olefins through a biomimetic approach[J]. Chem Eur J, 2013, 19(13): 4140–4145.
|
| [46] |
Oishi T, Yamaguchi K, Mizuno N. Conceptual design of heterogeneous oxidation catalyst: Copper hydroxide on manganese oxide-based octahedral molecular sieve for aerobic oxidative alkyne homocoupling[J]. ACS Catal, 2011, 1(10): 1351–1354.
|
| [47] |
Yoshii D, Jin X, Yatabe T, et al. Gold nanoparticles on OMS-2 forheterogeneously catalyzed aerobic oxidative α, β-dehydrogenation of β-heteroatom-substitutedketones[J]. Chem Commun, 2016, 52(99): 14314–14317.
|
| [48] |
Jin X, Yamaguchi K, Mizuno N. Gold-catalyzed heterogeneous aerobic dehydrogenative amination of α, β-unsaturated aldehydes to enaminals[J]. Angew Chem Int Ed, 2014, 53(2): 455–458.
|
| [49] |
Yamaguchi K, Wang Y, Oishi T, et al. Heterogeneously catalyzed aerobic cross-dehydrogenative coupling of terminal alkynes and monohydrosilanes by gold supported on OMS-2[J]. Angew Chem Int Ed, 2013, 52(21): 5627–5630.
|
| [50] |
Lucasa N, Gurralab L, Halligudi S. Efficacy of octahedral molecular sieves for green and sustainable catalytic reactions[J]. Molecular Catalysis, 2020, 490: 110966–110978.
|
| [51] |
Guo Yu-fang(郭玉芳), Huang Zhi(黄智). Research advance in catalytic performance of manganese oxide octahedral molecular sieve(氧化锰八面体分子筛催化性能研究进展)[J]. Ind Catal(China)(工业催化), 2017, 25(3): 1–6.
|
| [52] |
Zhang Nan(张楠), Li Jian-min(李建民), Liu Hai-jin(刘海津), et al. Preparation of Ag-Pd/g-C3N4 and selective oxidation of benzyl alcohol in aqueous solution(Ag-Pd/g-C3N4的制备及在水溶液中选择性氧化苯甲醇)[J]. J Mol Catal (China)(分子催化), 2019, 33(1): 75–82.
|
| [53] |
Li He-jian(李和健), Wu Zang-zang(吴藏藏), Zheng Li(郑丽), et al. Selective catalytic oxidation of benzyl alcohol over Cu-BTC and its derivative(Cu-BTC及其衍生物在苯甲醇选择氧化反应中的催化活性)[J]. J Mol Catal (China)(分子催化), 2017, 34(4): 341–347.
|
| [54] |
Cymes B, Almquist C, Krekeler M. Europium-doped cryptomelane: Multi-pathway synthesis, characterization, and evaluation for the gas phase catalytic oxidation of ethanol[J]. Appl Catal A-Gen, 2020, 589: 117310–117338.
|
| [55] |
Said S, Riad M. Oxidation of benzyl alcohol through eco-friendly processes using Fe-doped cryptomelane catalysts[J]. Solid State Sci, 2019, 94: 145–154.
|
| [56] |
Son Y, Makwana V, Howell A, et al. Efficient, catalytic, aerobic oxidation of alcohols oxidation using OMS-2 catalyst: A kinetic and isotope-labeling study[J]. Angew Chem Int Ed, 2001, 113: 4410–4413.
|
| [57] |
Makwana V, Son Y, Howell A, et al. The role of lattice oxygen in selective benzyl alcohol oxidation using OMS-2 catalyst: A kinetic and isotope-labeling study[J]. J Catal, 2002, 210(1): 46–52.
|
| [58] |
Yadav G, Mewada R. Selectivity engineering in the synthesis of value addedchemicals: Oxidation of 1-octanol to 1-octanal overnano-fibrous Ag-OMS-2 catalysts[J]. Chem Eng Res Des, 2012, 90(1): 86–97.
|
| [59] |
Nie J, Liu H. Efficient aerobic oxidation of 5-hydroxymethylfurfural to 2, 5-diformylfuran on manganese oxide catalysts[J]. J Catal, 2014, 316: 57–66.
|
| [60] |
Liu P, Duan J, Ye Q, et al. Promoting effect of unreducible metal doping on OMS-2 catalysts foras-phase selective oxidation of ethanol[J]. J Catal, 2018, 367: 115–125.
|
| [61] |
Deng Y, Zhang T, Au C, et al. Oxidation of p-chlorotoluene to p-chlorobenzaldehyde over manganese-based octahedralmolecular sieves of different morphologies[J]. Catal Commun, 2014, 43: 126–130.
|
| [62] |
Yamaguchi K, Kobayashi , Wang Y, et al. Green oxidative synthesis of primary amides from primary alcohols or aldehydes catalyzed by a cryptomelane-type manganese oxide-based octahedral molecular sieve, OMS-2[J]. Catal Sci Technol, 2013, 3(2): 318–327.
|
| [63] |
Mao Hui-ling(毛会玲), Wang Chen(王晨), Xue Yun(薛云), et al. Synthesis of radical decorated conjugated microporous polymers for selective oxidation of 5-hydroxymethylfurfural(自由基修饰共轭微孔聚合物应用于5-羟甲基糠醛选择性氧化)[J]. J Mol Catal (China)(分子催化), 2019, 33(3): 245–252.
|
| [64] |
Li X, Ma J, Jia X, et al. Al-doping promoted aerobic amidation of 5-hydroxymethylfurfuralto 2, 5-furandicarboxamide over cryptomelane[J]. ACS Sustainable Chem Eng, 2018, 6(6): 8048–8054.
|
| [65] |
Jia X, Ma J, Wang M, et al. Catalytic conversion of 5-hydroxymethylfurfuralinto 2, 5-furandiamidine dihydrochloride[J]. Green Chem, 2016, 18(4): 974–978.
|
| [66] |
Jia X, Ma J, Wang M, et al. Alkali α-MnO2/NaxMnO2 collaboratively catalyzedammoxidation-Pinner tandem reaction of aldehydes[J]. Catal Sci Technol, 2016, 6(20): 7429–7436.
|
| [67] |
Jia X, Ma J, Xia F, et al. Switching acidity on manganese oxide catalyst with acetylacetones for selectivity-tunable aminesoxidation[J]. Nat Commun, 2019, 10: 2338–2344.
|
| [68] |
Zhang Z, Wang F, Wang M, et al. Tert-Butyl hydroperoxide (TBHP)-mediatedoxidative self-coupling of amines to imines over a α-MnO2 catalyst[J]. Green Chem, 2014, 16(5): 2523–2527.
|
| [69] |
Sun Y, Ma H, Jia X, et al. A High-performance base-metal approach for the oxidative esterification of 5-hydroxymethylfurfural[J]. Chem Catal Chem, 2016, 8(18): 2907–2911.
|
| [70] |
Li X, Jia X, Ma J, et al. Catalytic amidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxamide over alkali manganese oxides[J]. Chin J Chem, 2017, 35(6): 984–990.
|
| [71] |
Jia X, Ma J, Xia F, et al. Carboxylic acid-modified metal oxide catalyst forselectivity-tunable aerobic ammoxidation[J]. Nat Commun, 2018, 9: 933–940.
|
| [72] |
Ferlin F, Marini A, Ascani N, et al. Heterogeneous manganese-catalyzed oxidase C-H/C-O cyclization to access pharmaceutically active compounds[J]. Chem Catal Chem, 2020, 12(2): 449–454.
|
| [73] |
Ferlin F, Hulst M, Santoro S, et al. Continuous flow/waste-minimized synthesis of benzoxazoles catalysed by heterogeneous manganese systems[J]. Green Chem, 2019, 21(19): 5298–5305.
|
| [74] |
Ferlin F, Navarro P, Gu Y, et al. Waste minimized synthesis of pharmaceuticallyactive compounds via heterogeneous manganese catalysed C-H oxidation in flow[J]. Green Chem, 2020, 22(2): 397–403.
|
| [75] |
Meng X, Bi X, Yu C, et al. Ball-milling synthesized hydrotalcite supported Cu-Mn mixed oxide under solvent-free conditions: An active catalyst for aerobic oxidative synthesis of 2-acylbenzothiazoles and quinoxalines[J]. Green Chem, 2018, 20(20): 4638–4644.
|
| [76] |
Meng X, Wang Y, Wang Y, et al. OMS-2-Supported Cu hydroxide-catalyzed benzoxazoles synthesis from catechols and amines via domino oxidation process at room temperature[J]. J Org Chem, 2017, 82(13): 6922–6931.
|
| [77] |
Bi X, Meng X, Chen G, et al. Manganese oxide catalyzed synthesis of anti-HIVN-substituted benzimidazoles via a one-pot multistep process[J]. Catal Commun, 2018, 116: 27–31.
|
| [78] |
Shen J, Meng X. Selective synthesis of pyrimidines from cinnamyl alcohols and amidines using the heterogeneous OMS-2 catalyst[J]. Catal Commun, 2020, 138: 105846–105850.
|
| [79] |
Shen J, Meng X. Base-free synthesis of 1, 3, 5-triazines via aerobic oxidation of alcohols and benzamidine over a recyclable OMS-2 catalyst[J]. Catal Commun, 2019, 127: 58–63.
|
| [80] |
Li B, Li C, Meng X, et al. Heterogeneous oxidative synthesis of quinazolines over OMS-2 under ligand-free conditions[J]. New J Chem, 2018, 42(19): 15985–15990.
|
| [81] |
Meng X, Wang Y, Chen B, et al. Environmentally-friendly heterogeneous catalytic system for oxidative synthesis of benzoxazoles at room temperature[J]. Org Pro Res Dev, 2017, 21(12): 2018–2024.
|
| [82] |
Meng X, Bi X, Wang Y, et al. Heterogeneous selective synthesis of 1, 2-dihydro-1, 3, 5-triazines from alcohols and amidines via Cu/OMS-2-catalyzed multistep oxidation[J]. Catal Commun, 2017, 89: 34–39.
|
| [83] |
Wang Y, Meng X, Chen G, et al. Direct synthesis of quinazolinones by heterogeneous Cu(OH)x/OMS-2 catalyst under oxygen[J]. Catal Commun, 2018, 104: 106–111.
|
| [84] |
Li B, Bi X, Meng X, et al. Synthesis of crystalline OMS-2 with urea hydrogen peroxide and its application in aerobic oxidation reactions[J]. Chem Select, 2019, 4(20): 6074–6079.
|
| [85] |
Wang M, Ma J, Yu M, et al. Oxidative coupling of anilines to azobenzenesusing heterogeneous manganese oxide catalysts[J]. Catal Sci Technol, 2016, 6(6): 1940–1945.
|
 2020, Vol. 34
2020, Vol. 34