2. 延边大学 药学院, 吉林 延吉 133000
2. Department of Pharmacy, Yanbian University, Yanji 133000, China
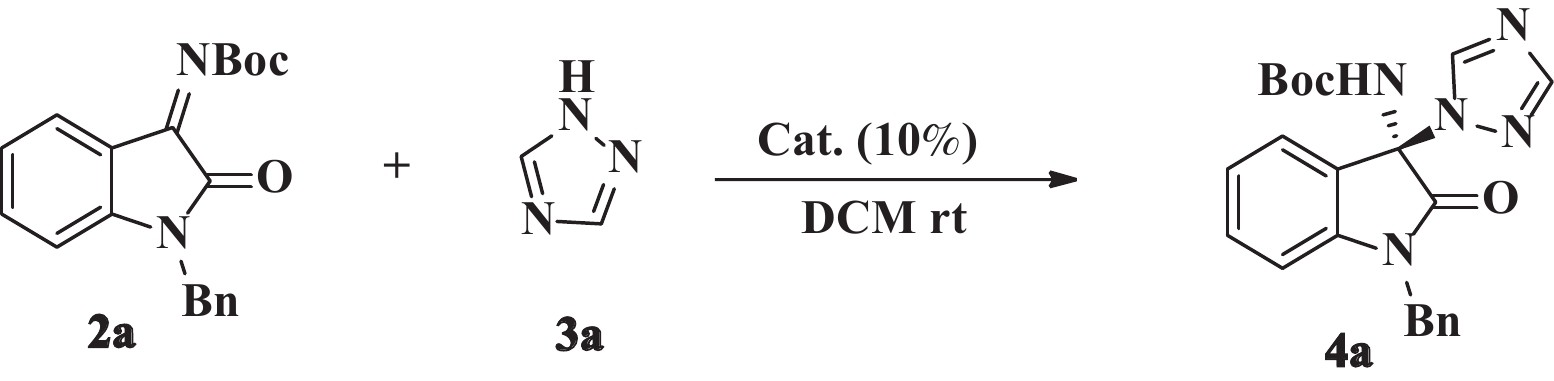
含有N,N'-缩酮-2-吲哚酮的骨架结构存在于很多天然产物和生物活性化合物中[1-13]. 然而, 由于N,N'-缩酮结构不稳定, 其合成方法的研究远不如O,O'-缩酮和O,N-缩酮化合物广泛[14-19]. 尤其是非环手性N,N'-缩酮化合物的制备更是有机合成领域中的难点. 靛红亚胺的不对称aza-Mannich反应是制备光学纯非环N,N’-缩酮-2-吲哚酮的有效途径[20-22]. 1,2,4-三氮唑衍生物在医药领域的应用广泛, 具有抗菌[23]、镇痛消炎[24]、抗肿瘤[25]等活性作用. 如伏立康唑、利巴韦林、来曲唑等. 因此, 以三氮唑为亲核试剂与靛红亚胺进行不对称aza-Mannich反应, 可以制备具有潜在生物活性的手性N,N'-缩酮-2-吲哚酮衍生物. 2019年, Zhang[21]等报道了金鸡纳碱硫脲催化三氮唑与靛红亚胺的不对称Mannich反应, 以90%~99%的产率和86%~97%的立体选择性获得具有C3位N,N'-缩酮结构的3-氨基-2-吲哚酮衍生物. 目前, 三氮唑衍生物与靛红亚胺的反应仅有上述1篇文献报道, 催化剂为金鸡纳碱衍生物. 近年来, Takemoto型催化剂被广泛应用于靛红亚胺的不对称Mannich反应[22,26-29], 我们将Takemoto型(硫)脲类衍生物催化剂1a−1l, 应用于aza-Mannich反应(图1), 以期拓宽该反应的催化剂类型.

|
图 1 手性Takemoto型(硫)脲催化剂1a−1l的结构 Fig.1 The structure of chiral Takemoto′s (thio)urea catalysts 1a−1l |
催化剂1a−1l购买于上海大赛璐试剂有限公司;硅胶GF254薄层板及柱色谱分离用粒径0.071~0.050 mm硅胶购买于山西诺泰生物科技有限公司; 其他分析纯试剂通过市售渠道购买; 1H NMR和13C NMR光谱通过Bruker Avance-500型核磁共振谱仪(德国Bruker 公司)测定; 以氘代CDCl3为溶剂, 以未氘代的CHCl3为内标(分别为氢谱7.26和碳谱77.0); 高分辨质谱HRMS的测定使用Triple TOF 5600+型质谱仪(美国Sciex 公司); 旋光值通过A28579-T-CG APIII型自动旋光仪(美国Rudolph 公司)测定; 对映体过量值(ee)的测定使用LC-20A高效液相色谱仪(日本岛津公司)及Daicel ChiralpakAS-H手性色谱柱(4.6 mm×250 mm,日本大赛璐公司).
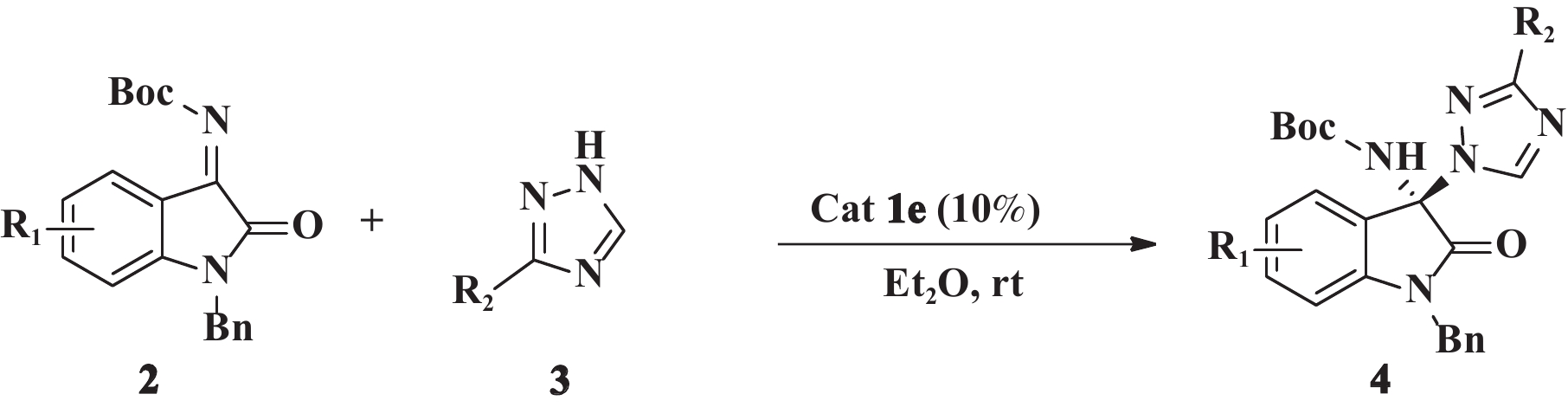
1.2 不对称aza-Mannich反应的一般操作步骤将靛红亚胺2(0.1 mmol), 1,2,4-三氮唑3(0.2 mmol)和催化剂1(10%(摩尔分数)), 乙醚(Et2O)1 mL加入10 mL干燥反应管中, 室温下搅拌反应12~24 h, TLC监测, 展开剂(Hex∶EA = 1∶1), 反应结束后, 经快速柱层析分离纯化(Hex∶EA = 2∶1), 得到目标产物4a−4l. 其中化合物4c、4d、4f−4l为新化合物, 其1H NMR、13C NMR、HRMS、HPLC、熔点及比旋光值如下:
(R)-(1-苄基-5-氯-3-(1H-1,2,4-三唑-1-)-2-吲哚酮-3-)氨基甲酸叔丁酯4c: 白色固体; 81% yield; mp: 136.6~138.2 ℃; 1H NMR (500 MHz, CDCl3) δ 8.24 (s, 1H), 8.00 (s, 1H), 7.91 (d, J = 2.0 Hz, 1H), 7.37-7.26 (m, 6H), 6.71 (d, J = 8.5 Hz, 1H), 6.17 (s, 1H), 4.95 (q, J = 16.0 Hz, 2H), 1.43 (s, 9H). HR-ESI-MS m/z: 462.1411 [M+Na]+ (calcd for C22H22ClN5O3Na, 462.1415);
(R)-(1-苄基-7-溴-3-(1H-1,2,4-三唑-1-)-2-吲哚酮-3-)氨基甲酸叔丁酯4d: 白色固体; 82% yield; mp: 68.9~71.2 ℃; 1H NMR (500 MHz, CDCl3) δ 8.16 (s, 1H), 8.00 (s, 1H), 7.85 (dd, J = 7.5, 1.0 Hz, 1H), 7.51 (dd, J = 8.0, 1.0 Hz, 1H), 7.31 (dd, J = 8.0, 6.5 Hz, 2H), 7.28-7.20 (m, 3H), 7.09-7.01 (m, 1H), 6.21 (s, 1H), 5.45 (q, J = 16.5 Hz, 2H), 1.41 (s, 9H).13C NMR (125 MHz, CDCl3) δ 170.9, 153.1, 152.8, 142.3, 140.3, 137.4, 136.2, 128.7, 128.4, 127.4, 126.2, 126.1, 125.1, 103.3, 82.2, 72.2, 45.4, 28.1. HR-ESI-MS m/z: 506.0906 [M+Na]+ (calcd for C22H22BrN5O3Na, 506.0902);
(R)-(1-苄基-3-(3-甲基-1H-1,2,4-三唑-1-)-2-吲哚酮-3-)氨基甲酸叔丁酯4e: 白色固体; 90% yield; mp: 193.3~195.8 ℃; 1H NMR (500 MHz, CDCl3) δ 7.93 (s, 1H), 7.84-7.79 (m, 1H), 7.35-7.25 (m, 7H), 7.14 (td, J = 7.5, 1.0 Hz, 1H), 6.78 (d, J = 8.0 Hz, 1H), 6.25 (s, 1H), 4.96 (dd, J =47.5, 16.0 Hz, 2H), 2.40 (s, 3H), 1.39 (s, 9H). HR-ESI-MS m/z: 442.1957 [M+Na]+ (calcd for C23H25rN5O3Na, 442.1953);
(R)-(1-苄基-5-氟-3-(3-甲基-1H-1,2,4-三唑-1-)-2-吲哚酮-3-)氨基甲酸叔丁酯4f: 白色固体; 80% yield; mp: 179.2~182.7 ℃; 1H NMR (500 MHz, CDCl3) δ 8.98 (s, 1H), 8.71 (s, 1H), 7.43-7.35 (m, 3H), 7.32 (dd, J = 10.0, 4.5 Hz, 2H), 7.28-7.24 (m, 1H), 7.18 (td, J = 9.0, 2.5 Hz, 1H), 6.92 (dd, J = 8.5, 4.0 Hz, 1H), 4.99 (d, J = 16.0 Hz, 1H), 4.91 (d, J = 16.0 Hz, 1H), 2.19 (s, 3H), 1.30 (s, 9H), 13C NMR (125 MHz, CDCl3) δ 170.8, 161.2, 160.4, 158.4, 154.5, 144.8, 139.8, 136.3, 129.6, 129.5 (d, J = 22.4 Hz), 128.4, 128.1, 117.9, 117.7, 113.6, 113.4, 111.8 (d, J = 7.7 Hz), 81.3, 74.3, 44.1, 28.7, 14.6. HR-ESI-MS m/z: 460.1761 [M+Na]+ (calcd for C23H24FN5O3Na, 460.1768);
(R)-(1-苄基-5-氯-3-(3-甲基-1H-1,2,4-三唑-1-)-2-吲哚酮-3-)氨基甲酸叔丁酯4g: 白色固体; 81% yield; mp: 172.1~174.4 ℃; 1H NMR (500 MHz, CDCl3) δ 8.02 (s, 1H), 7.82 (d, J = 2.0 Hz, 1H), 7.35-7.26 (m, 6H), 6.68 (d, J = 8.5 Hz, 1H), 6.20 (d, J = 6.0 Hz, 1H), 5.00 (d, J = 16.0 Hz, 1H), 4.91 (d, J = 16.0 Hz, 1H), 2.40 (s, 3H), 1.41 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 169.8, 162.4, 153.2, 142.3, 141.2, 134.1, 131.2, 129.3, 129.0, 128.1, 127.3, 127.1, 126.9, 111.2, 82.0, 72.5, 44.6, 28.1, 14.1; HR-ESI-MS m/z: 476.1465 [M+Na]+ (calcd for C23H24ClN5O3Na, 476.1460);
(R)-(1-苄基-5-溴-3-(3-甲基-1H-1,2,4-三唑-1-)-2-吲哚酮-3-)氨基甲酸叔丁酯4h: 白色固体; 86% yield; mp: 168.1~172.2 ℃; 1H NMR (500 MHz, DMSO) δ 9.00 (s, 1H), 8.73 (s, 1H), 7.66 (d, J = 2.0 Hz, 1H), 7.51 (dd, J = 8.5, 2.0 Hz, 1H), 7.38 (d, J = 7.0 Hz, 2H), 7.33-7.29 (m, 2H), 7.26 (dd, J = 8.5, 6.0 Hz, 1H), 6.89 (d, J = 8.5 Hz, 1H), 4.99 (d, J = 16.0 Hz, 1H), 4.91 (d, J = 16.0 Hz, 1H), 2.19 (s, 3H), 1.30 (s, 9H); 13C NMR (125 MHz, DMSO) δ 170.5, 161.2, 154.4, 144.7, 142.8, 136.1, 134.1, 130.1, 129.3, 128.3, 128.2, 128.0, 115.5, 112.7, 81.3, 74.0, 44.1, 28.6, 14.5. HR-ESI-MS m/z: 520.0955 [M+Na]+ (calcd for C23H24BrN5O3Na, 520.0961);
(R)-(1-苄基-5-甲基-3-(3-甲基-1H-1,2,4-三唑-1-)-2-吲哚酮-3-)氨基甲酸叔丁酯4i: 白色固体; 85% yield; mp: 174.0~176.7 ℃; 1H NMR (500 MHz, DMSO) δ 8.89 (s, 1H), 8.64 (s, 1H), 7.39 (d, J = 7.5 Hz, 2H), 7.30 (dd, J = 10.0, 5.0 Hz, 3H), 7.25 (t, J = 7.0 Hz, 1H), 7.10 (dd, J = 8.0, 1.0 Hz, 1H), 6.77 (d, J = 8.0 Hz, 1H), 4.96 (d, J = 16.0 Hz, 1H), 4.86 (d, J = 16.0 Hz, 1H), 2.24 (s, 3H), 2.18 (s, 3H), 1.29 (s, 9H); 13C NMR (125 MHz, DMSO) δ 170.9, 161.0, 144.6, 141.2, 136.6, 133.0, 131.6, 129.3, 128.3, 128.1, 126.0, 110.5, 81.0, 74.6, 44.0, 28.8, 21.6, 14.6. HR-ESI-MS m/z: 456.2012 [M+Na]+ (calcd for C24H27N5O3Na, 456.2019);
(R)-(1-苄基-5-甲氧基-3-(3-甲基-1H-1,2,4-三唑-1-)-2-吲哚酮-3-)氨基甲酸叔丁酯4j: 白色固体; 85% yield; mp: 159.4~162.5 ℃; 1H NMR (500 MHz, CDCl3) δ 7.95 (s, 1H), 7.46 (d, J = 2.5 Hz, 1H), 7.37-7.26 (m, 5H), 6.83 (dd, J = 8.5, 2.5 Hz, 1H), 6.67 (d, J = 8.5 Hz, 1H), 6.23 (s, 1H), 4.97 (d, J = 16.0 Hz, 1H), 4.89 (d, J = 16.0 Hz, 1H), 3.76 (s, 3H), 2.40 (s, 3H), 1.40 (s, 9H); 13C NMR (125 MHz, CDCl3) δ 169.9, 156.6, 153.3, 142.4, 135.9, 134.6, 128.9, 127.9, 127.1, 116.2, 113.7, 110.8, 81.7, 72.9, 55.8, 44.5, 28.1, 14.2; HR-ESI-MS m/z: 472.1961 [M+Na]+ (calcd for C24H27N5O3Na, 472.1967);
(R)-(1-苄基-7-氟-3-(3-甲基-1H-1,2,4-三唑-1-)-2-吲哚酮-3-)氨基甲酸叔丁酯4k: 白色固体; 83% yield; mp: 182.0~184.2 ℃; 1H NMR (500 MHz, CDCl3) δ 7.91 (s, 1H), 7.59 (dd, J = 5.5, 3.0 Hz, 1H), 7.39-7.27 (m, 5H), 7.12-7.06 (m, 2H), 6.24 (s, 1H), 5.09 (q, J = 15.5 Hz, 2H), 2.39 (s, 3H), 1.39 (s, 9); 13C NMR (125 MHz, CDCl3) δ 170.0, 162.4, 153.1, 148.6, 146.6, 142.3, 135.9, 128.7, 128.2, 128.0 (d, J = 32.7 Hz), 127.4, 124.5 (d, J = 6.2 Hz), 122.6, 119.5 (d, J = 19.4 Hz), 81.9, 72.6, 46.2, 28.1, 14.1. HR-ESI-MS m/z: 460.1761 [M+Na]+ (calcd for C23H24FN5O3Na, 460.1766);
(R)-(1-苄基-7-溴-3-(3-甲基-1H-1,2,4-三唑-1-)-2-吲哚酮-3-)氨基甲酸叔丁酯 4l: 白色固体; 82% yield; mp: 168.9~171.4 ℃; 1H NMR (500 MHz, CDCl3) δ 7.92 (s, 1H), 7.76 (dd, J = 7.5,1.0 Hz, 1H), 7.49 (dd, J = 8.0, 1.0 Hz, 1H), 7.34-7.23 (m, 5H), 7.03 (t, J = 8.0 Hz, 1H), 6.23 (s, 1H), 5.45 (q, J = 16.5 Hz, 2H), 2.39 (s, 3H), 1.40 (s, 9H);13C NMR (125 MHz, CDCl3) δ 171.0, 162.5, 153.1, 142.3, 140.4, 137.3, 136.4, 128.7, 127.3, 126.2, 125.7, 125.0, 103.3, 82.0, 72.0, 45.3, 28.1, 14.1. HR-ESI-MS m/z: 520.0955 [M+Na]+ (calcd for C23H24BrN5O3Na, 520.0950);
将催化剂1a−1l应用于靛红亚胺2a与1,2,4-三氮唑3a的不对称aza-Mannich反应, 考察催化剂的催化性能. 根据文献报道的最优条件[21], 选用二氯甲烷为溶剂, 10%(摩尔分数)催化剂用量, 室温反应12 h. 反应结果见表1.
| 表 1 靛红亚胺与1,2,4-三氮唑的不对称aza-Mannich反应的结果a Table 1 Asymmetric aza-Mannich reaction of isatin imine with 1,2,4-triazolea |
由表1结果可以看出12种催化剂1a−1l在二氯甲烷中均能顺利催化靛红亚胺和1,2,4-三氮唑的不对称aza-Mannich反应, 以75%~87%的产率获得目标产物. 其中(R,R)-N-吡咯硫脲催化剂1e表现出最好的的催化性能, 得到61% ee(Entry 5). 通过测定其旋光值, 并与文献的数据进行比较[21], 确定主要产物的绝对构型为R. 奇怪的是, 当以(S,S)-N-吡咯硫脲催化剂1f催化该反应, 得到了消旋的产物. 此外, 当硫脲催化剂环己胺N上的取代的基团更大时, 不利于催化剂的诱导作用, 所得相应目标产品的立体选择性均有所下降(Entry 7−9 vs Entry 5). 综上, 筛选出最优催化剂为1e.
2.2 反应条件的优化将催化剂1e应用于靛红亚胺2a和1,2,4-三氮唑3a的不对称aza-Mannich反应中. 通过考察不同种类溶剂、温度、催化剂负载量等条件对催化效能的影响, 以期优化反应条件, 提高反应的立体选择性. 结果见表2.
| 表 2 1e催化靛红亚胺与苯胺的不对称aza-Mannich反应的条件筛选a Table 2 Screening of reaction condition for the asymmetric aza-Mannich reaction catalyzed by 1ea |
由表2可得出以下结果:(1)溶剂对反应的产率和立体选择性有影响:以乙醚为溶剂时获得了最好的对映选择性(86% ee, Entry 2)而在乙腈和二甲苯的条件下, 立体选择性分别下降至7% ee和9% ee (Entry 6, 8); (2) 温度对反应立体选择性有一定的影响: 当温度降至0 ℃时, 反应的产率和ee值均有所下降(Entry 9 vs Entry 2). 当温度继续降至−10 ℃时, 反应速度变得更慢, 产品的立体选择性下降至69% ee (Entry 10); (3) 将催化剂用量增加至20%(摩尔分数), 产品的ee值和产率没有得到提高(Entry 11 vs Entry 2), 而将用量降至5%(摩尔分数), 反应的产率和立体选择性均有所下降(Entry 12 vs Entry 2); (4) 当稀释反应浓度一倍, 即溶剂用量增至2 mL时, 反应的产率和立体选择性均有所下降(Entry 13 vs Entry 2); (5) 加入0.4 nm分子筛, 产品的ee值明显下降至70%(Entry 14 vs Entry 2). 基于以上结果, 筛选出的最佳反应体系为: 10%(摩尔分数)催化剂1e, 1 mL Et2O, 室温反应.
2.3 普适性的考察将上述筛选的最佳催化剂体系用于8种不同取代靛红亚胺和2种1,2,4-三氮唑应的不对称aza-Mannich反应中, 扩展该反应底物的范围, 考察催化剂体系对反应的普适性, 结果见表3.
| 表 3 不同取代靛红亚胺与三氮唑的不对称aza-Mannich反应a Table 3 Generility of the enantioselective aza-Mannich reaction of isatin derived imines with 1,2,4-triazolea |
由表3可以看出:在最优反应条件下, 多种取代靛红亚胺和1,2,4-三氮唑及3-甲基三氮唑的不对称aza-Mannich反应均能够顺利进行, 以77%~90%的产率获得相应的目标产物4a−4m. 其中苯环上没有取代基的苄基靛红亚胺2a为底物与不同三氮唑的反应得到了最佳的立体选择性, 分别为86% ee和99% ee(Entry 1, Entry 5). 与1,2,4-三氮唑相比, 以3-甲基-1,2,4-三氮唑为底物, 明显能够提高反应的立体选择性(Entry 5−7,12 vs Entry1−4). 综上, 靛红亚胺上取代基的种类和位置以及三氮唑结构上的甲基对反应的立体选择性均有影响, 机理尚不清楚, 有待于进一步研究.
根据得到产品的绝对构型, 提出可能的过渡态如图2所示: 双功能催化剂1e的硫脲结构通过双氢键定位和活化靛红亚胺, 同时, 催化剂的叔胺氮与三氮唑形成氢键, 进而去质子活化的三氮唑从re-面进攻亚胺基, 得到R构型产品.
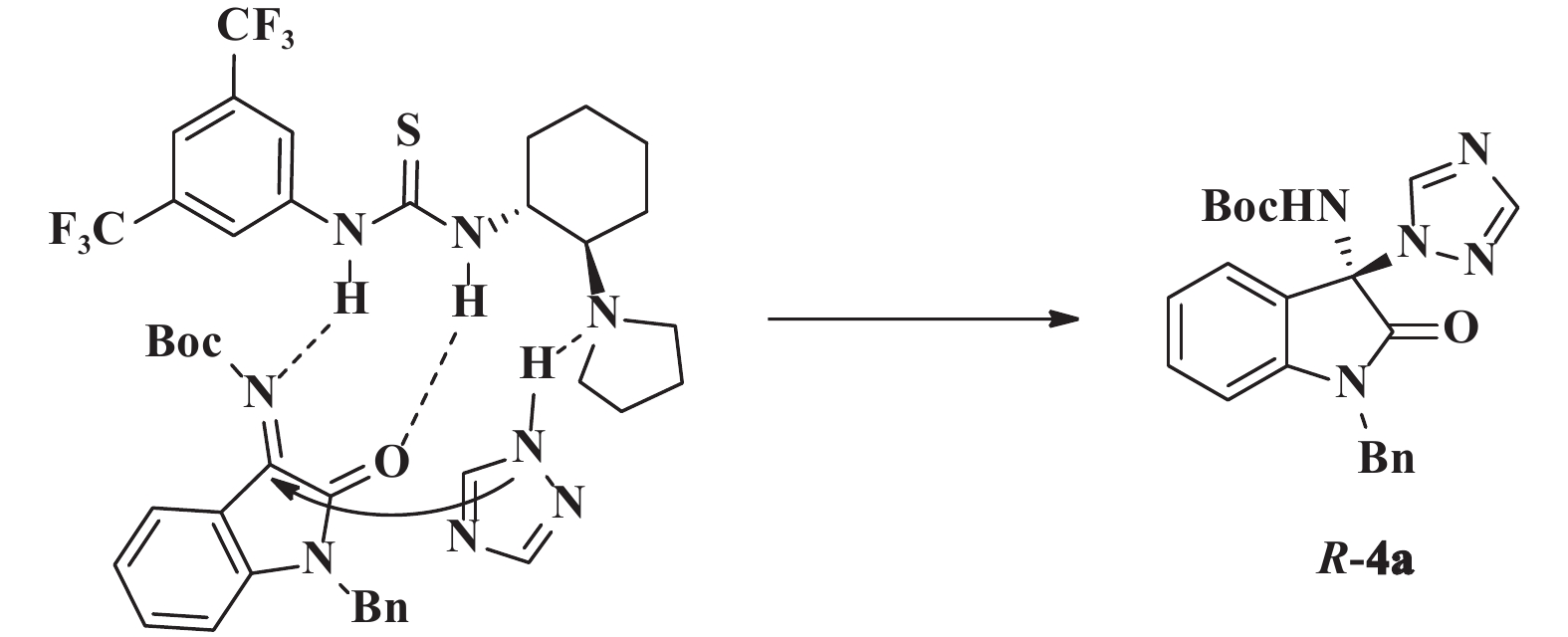
|
图 2 可能的过渡态模型 Fig.2 Proposed transition state model |
我们将12种Takemoto型(硫)脲催化剂应用于苄基靛红亚胺与1,2,4-三氮唑的不对称aza-Mannich反应中. 通过考察催化剂结构、用量、溶剂的种类、反应液浓度、温度及分子筛等条件对该反应的立体选择性的影响, 筛选出最优催化剂条件, 并应用于不同取代靛红亚胺的和不同取代苯胺不对称aza-Mannich反应, 以最高达99%的对映选择性获得手性3-N,N'-缩酮-2-吲哚酮衍生物. 扩展了该反应中催化剂的类型. 但是, 反应普适性还有待于提高.
| [1] |
Synthesis of novel spiro[pyrazolo[4,3-d]pyrimidinones and spiro[benzo-[4,5]thieno[2,3-d] pyrimidine-2,3′-indoline] 2′,4(3H)-diones and their evaluation for anticancer activity[J]. Med Chem Lett, 2017, 27(6): 1446–1450.
DOI:10.1016/j.bmcl.2017.01.088 |
| [2] |
Synthesis of novel 7-aryl and 7-spiropyrazolo[4′,3′: 5,6] pyrido[2,3-d]pyrimidine derivatives and their study as AChE inhibitors[J]. Mol Divers, 2017, 21(4): 943–955.
DOI:10.1007/s11030-017-9774-3 |
| [3] |
Indole Alkaloids from Cephalanceropsis gracilis[J]. J Nat Prod, 2006, 69(10): 1467–1470.
DOI:10.1021/np060395l |
| [4] |
Synthesis and structure elucidation of novel fused 1,2,4-triazine derivatives as potent inhibitors targeting CYP1A1 activity[J]. Bioorg Med Chem, 2012, 20(8): 2624–2637.
DOI:10.1016/j.bmc.2012.02.041 |
| [5] |
Spiro heterocycles as potential inhibitors of SIRT1: Pd/C-mediated synthesis of novel N-indolylmethyl spiroindoline-3,2′-quinazolines[J]. Bioorg Med Chem Lett, 2013, 23(5): 1351–1357.
DOI:10.1016/j.bmcl.2012.12.089 |
| [6] |
Regioselective synthesis of biologically important scaffold spiro [Indole- Perimidines]: An antitumor agents[J]. Lett Org Chem, 2007, 4(5): 378–383.
DOI:10.2174/157017807781212175 |
| [7] |
Asymmetric synthesis of 2,3-dihydro-2- arylquinazolin- 4-ones: Methodology and application to a potent fluorescent tubulin inhibitor with anticancer activity[J]. J Med Chem, 2008, 51(15): 4620–4631.
DOI:10.1021/jm800271c |
| [8] |
Unprecedented lewis acid catalyzed one-pot, three-component synthesis and evaluation of bioactive property of 1-(2-oxo-3-(2-phenylhydrazinyl) indolin-3-yl) pyrrolidine-2,5-dione[J]. Int J Pharm Sci Rev Res, 2015, 35(1): 12–15.
|
| [9] |
Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents[J]. Angew Chem, Int Ed, 2007, 46(46): 8748–8758.
DOI:10.1002/anie.200701342 |
| [10] |
The asymmetric intramolecular heck reaction in natural product total synthesis[J]. Chem Rev, 2003, 103(8): 2945–2964.
DOI:10.1021/cr020039h |
| [11] |
Catalytic asymmetric synthesis of oxindoles bearing a tetrasubstituted stereocenter at the C-3 position[J]. Adv Synth Catal, 2010, 352(9): 1381–1407.
DOI:10.1002/adsc.201000161 |
| [12] |
Transition-metal-mediated routes to 3, 3-disubstituted oxindoles through anilide cyclisation[J]. Eur J Org Chem, 2011, 2011(34): 6821–6841.
DOI:10.1002/ejoc.201100836 |
| [13] |
Recent progress in enantioselective synthesis of C3-functionalized oxindoles: Rare earth metals take action[J]. Chem Sci, 2012, 3(2): 327–334.
DOI:10.1039/C1SC00544H |
| [14] |
a. Wang Y M, Zhang H H, Li C, et al. Catalytic asymmetric chemoselective 1,3-dipolar cycloadditions of an azomethine ylide with isatin-derived imines: Diastereo- and enantioselective construction of a spiro [imidazolidine-2,3′-oxindole] framework[J]. Chem Commun, 2016, 52(9): 1804−1807.b. Tian Jing(田 静), Sun Wei(孙 伟). Advances in biomimetic asymmetric oxidation catalyzed by N4 metal complexes(四氮金属配合物仿生催化不对称氧化研究进展)[J]. J Mol Catal(China)(分子催化), 2023, 37(1): 73−93.c. Ge Wei-wei(葛伟伟), Kong Fan-hua(孔凡华), Huang Li-hua(黄力华). Asymmetric synthesis of α-arylpropanol compounds by chiral phosphoramide compounds catalysts(手性磷酰胺类化合物不对称催化合成α-芳基丙醇类化合物)[J]. J Mol Catal(China)(分子催化), 2022, 36(1): 22−31.
|
| [15] |
Diastereo- and enantioselective construction of spirooxindole scaffolds through a catalytic asymmetric [3+3] cycloaddition
[J]. Org Biomol Chem, 2017, 15(22): 4794–4797.
DOI:10.1039/C7OB01059A |
| [16] |
Recent developments in the stereoselective synthesis of nitrogen - containing heterocycles using N-acylimines as reactive substrates[J]. Adv Synth Catal, 2016, 358(23): 3657–3682.
DOI:10.1002/adsc.201600644 |
| [17] |
Nonanomeric spiroketals in natural products: Structures, sources and synthetic strategies[J]. Chem Rev, 2005, 105(12): 4406–4440.
DOI:10.1021/cr050559n |
| [18] |
Synthetic approaches to spiroaminals[J]. Eur J Org Chem, 2008, 2008(26): 4391–4399.
DOI:10.1002/ejoc.200800371 |
| [19] |
Controlling regioselectivity in the enantioselective N-alkylation of indole analogues catalyzed by dinuclear zinc-prophenol[J]. Angew Chem Int Ed, 2017, 56(35): 10451–10456.
DOI:10.1002/anie.201705315 |
| [20] |
Highly enantioselective synthesis of acyclic N,N′-acetals by chiral urea derived from quinine catalyzed the addition of aryl amines to isatin-derived ketimines
[J]. Org Lett, 2019, 21(14): 5719–5724.
DOI:10.1021/acs.orglett.9b02098 |
| [21] |
Enantioselective construction of 3-substituted 3-amino-2-oxindoles containing N,N-ketal skeleton via organocatalyzed aza-Addition of isatin imines
[J]. Org Biomol Chem, 2019, 17(36): 8374–8378.
DOI:10.1039/C9OB01870K |
| [22] |
Jiang Hui-ting(姜惠婷), Li Xin-lu(李昕潞), Jin Yan(金 言), et al. Chiral tertiary amine urea catalyed enantioselective Aza-mannich reaction of isatin ketimines and aryl amines(手性叔胺脲催化靛红亚胺与苯胺的不对称aza-Mannich反应)[J]. Chem Res Appl(化学研究与应用), 2023, 35(3): 640−644.
|
| [23] |
Studies on the synthesis and antibacterial activity of 3,6-disubstituted 1,2,4-triazolo [3,4-b]1,3,4-thiadiazoles[J]. Eur J Med Chem, 2012, 47(2): 580–584.
|
| [24] |
Syntheses and evaluation of anti - inflammatory, analgesic and ulcerogenic activities of 1,3,4-oxadiazole and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole derivatives[J]. J Enzym Inhib Med Chem, 2012, 27(5): 658–665.
DOI:10.3109/14756366.2011.606543 |
| [25] |
Discovery of potent DOT1L inhibitors by AlphaLISA based high throughput screening assay[J]. Bioorg Med Chem, 2018, 26(8): 1751–1758.
DOI:10.1016/j.bmc.2018.02.020 |
| [26] |
Ričko S, Meden A, Ciber L, et al. Construction of vicinal tetrasubstituted stereogenic centers via a mannich-type organocatalyzed addition of Δ2-pyrrolin-4-ones to isatin imines[J]. Adv Synth Catal, 2018, 360(6): 1072−1 076.
|
| [27] |
Thiourea catalyzed domino michael mannich [3+2] cycloadditions: A strategy for the asymmetric synthesis of 3,3’ pyrrolidinyl dispirooxindoles[J]. Synlett, 2017, 8(20): 2876–2880.
|
| [28] |
Catalytic diastereo- and enantioselective vinylogous mannich reaction of alkylidenepyrazolones to isatin-derived ketimines[J]. Org Lett, 2021, 23(19): 7391–7395.
DOI:10.1021/acs.orglett.1c02571 |
| [29] |
Squaramide- catalyzed asymmetric mannich reactions of azlactones with isatin-derived ketimines: Access to α,β-diamino acid derivatives bearing vicinal quaternary stereocenters
[J]. J Org Chem, 2020, 85(5): 3894–3901.
DOI:10.1021/acs.joc.9b03000 |
 2023, Vol. 37
2023, Vol. 37