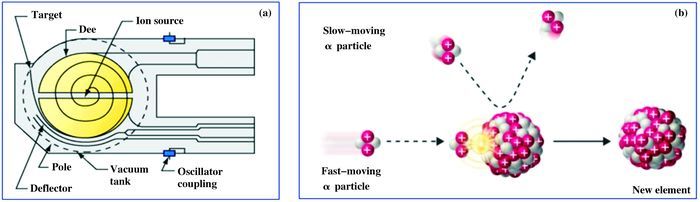
Nuclear transmutation is a process in which one element or its isotope is converted into another element or isotope[1]. Modern physics studies show that element transmutation can take place either by natural process or artificial process. The natural transmutations of radioactive elements (such as uranium and plutonium) can yield other radioactive elements or isotopes, while produce huge amount of energy and some possible dangerous and harmful matters to human beings due to the strong radioactive emission[2-4]. In 1919, Ru-therford realized the first artificial transmutation from nitrogen into oxygen by using alpha particle bombing to nitrogen atoms, known as 14N + α → 17O + p[2-4]. In 1932, John Cockcroft and Ernest Walton fulfilled an artificial nuclear reaction by 7Li bombing with accelerated protons to split the Li nucleus into two alpha particles, known as "splitting the atom"[5]. In 1938, Otto Hahn et al. discovered artificial transmutation reaction of heavy elements, which could be described by formulas as follows: 197Au + n → 198Au (half-life 2.7 days) → 198Hg + n → 199Hg + n → 200Hg + n → 201Hg + n → 202Hg + n → 203Hg (half-life 47 days) → 203Ti + n → 204Ti (half-life 3.8 years) → 204Pb[6-8]. Scientists also successfully achieved transmutation by accelerating atoms and some subatomic particles (e.g. protons, electrons and positrons) with high speed (near light speed) in particle accelerators (Scheme 1 (a))[6-9]. Owning to drastically collision, atom transmutations can take place when they "throw" some subatomic particles outside from their nucleus or "take in" some subatomic particles into their nucleus (Scheme 1 (b)). Nevertheless, inducing transmutation reaction via colliding method requires highly on accelerator equipment, vast power supply and cost.
|
Scheme 1 Schematic diagram of (a) a cyclotron particle accelerators (D-shaped magnets (called Dee)) and (b) A typical atoms transmutations process |
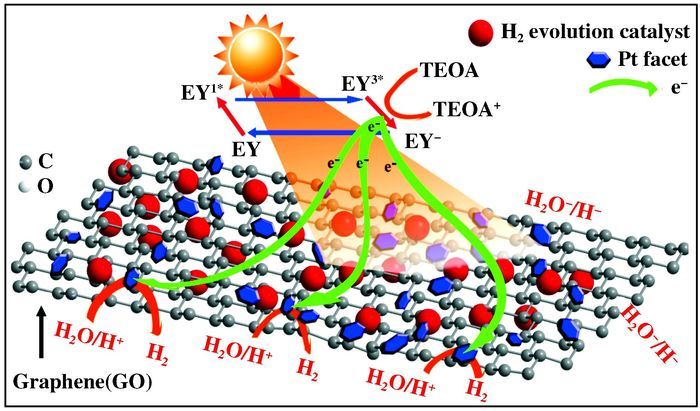
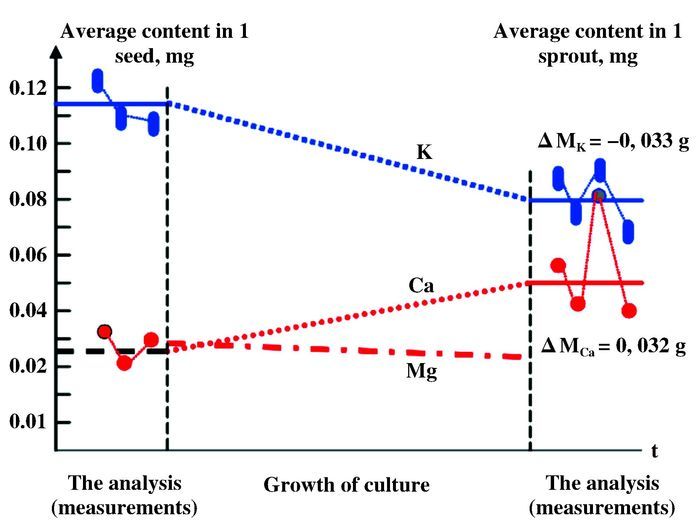
Besides natural and artificial transmutation, a large number of experiments certified that transmutation can be initiated in "mild" reactive systems under certain circumstances, like in metabolism processes of vegetal and animal organisms over the past two centuries[10-15]. In other words, some living organisms maintain capability to transmute some elemental atoms to the different ones while discharging energy slowly. To investigate transmutation in biological systems, Kervran et al. investigated potassium and calcium content variation during the growth of 840 seeds and 403 sprouts. Based on recorded data (illustrated in Scheme 2), they proposed potassium may transmute into calcium during the process of seeds growing, which could be represented in reaction formula: 39K + 1p = 40Ca + E[10-13]. Some other elemental transmutations were also reported, such as sodium to magnesium (1123Na + n → 1124Na* → 1224Mg + e- + ve*) and manganese to iron (2555Mn + n → 2556Mn* → 2656Fe + e- + ve*)[14]. Some studies point out bio-transmutations is associated with ATP hydrolysis processes catalyzed by ATPase in biological bodies[15]. Those investigations imply that the elemental transmutation is essential to maintain a balance of certain elements in the biological bodies, which is critical to organism growth.
|
Scheme 2 Changes in K and Ca contents in the seeds and sprouts The left and the right parts of the figure show the measured results by three series[10, 13] |
Despite of those achievements, many mysteries are still uncovered for transmutations in "mild" reactive systems. Some researchers are puzzled about bio-transmutation, for they believe biological system can not provide sufficient energies to break strong forces that keep the nucleons within their well-determined structure. On the contrary, Kervran et al. provided many experimental evidences for bio-transmutations, and proposed such transmutation occurrence is due to enzymes which could act as reaction catalysts in biolo-gical body[10-14]. Other researchers considered that transmutation in "mild" reactive system might due to protons tunneling through nuclei[15], otherwise the energy released from reactive systems would be so huge that it would set everything on fire all around. Moreover, some scientists ascribed transmutations in "mild" reactive systems to natural background radiations (cosmic radiation). That seems reasonable if considering there are so many particles in cosmic radiation which can pass through our body, including about 50 muons from cosmic rays, 10 million neutrinos coming directly from the Big Bang, 30 million photons from the cosmic microwave background, and 300 trillion neutrinos from the Sun in every second[16-17]. Based on "neutral current hypothesis" proposed by Weinberg, transmutation could be triggered when nuclei interacted with those particles, like positive β-decay process, a neutron capturing a neutrino and transmuting into a proton and an electron (β radiation) with the emission of an anti-neutrino, vice versa[14-15, 19-20]. The process is associated with heat variation, and can be described as[19]:
n(0) + v(0) → p+ + e- + v{anti}(0) (atom number plus 1, namely, Z+1)
or
p+ + e- + v{anti}(0) → n(0) + v(0) (atom number minus 1, namely, Z-1)
where n(0) referring to neutron, v(0) referring to neutrino, v{anti}(0) to anti-neutrino, p+ to proton, and e- to electron.
Up to now, there is no unified point of view on the mechanism of elemental transmutations in "mild" reactive systems. Unveiling that mystery is extremely meaningful for understanding how life evolution and surviving on earth, as well as providing safer and low-cost way to make use of nucleus energy. Given that, more explorations have to be done to obtain abundant experimental data and establishing convincing theory based on those data.
In this work, we fulfill the transmutation of potassium element to calcium during photochemical reaction of hydrogen evolution (HER). Photochemical HER reaction system is a bionic system which simulates the behavior of plants in nature to absorb and convert solar energy for hydrogen production[21-24]. Potassium was chosen as the target transmutation element in this work, taking into account the essential roles of potassium and calcium in biological bodies. Photochemical HER reaction system was chosen to provide a mild nuclear reaction environment, inspired by a recent discovery of our group that deuterium and helium were generated from protons by LENR in that system[25-26].
It is interesting and exotic to find that the concentration of calcium elements increased during the photochemical HER reaction in the presence of potassium. The results indicate that the increasing of calcium elements originated from nuclear transmutation by LENR. To our best knowledge, by far, there have been little literal research reports focusing on potassium transmutation to calcium occurring in photochemical HER reactive system. We know the surprising results raise many questions, and more experiments are required on this topic. Nevertheless, the obtained results provide evidences for scientists to uncover and unify the mechanism of elemental transmutations in "mild" reactive systems, which might be closely related to H atoms with electro-negativity (H-) generated in reaction system.
1 Materials and Experiments 1.1 MaterialsAll chemicals were purchased and used without further purification. Potassium chloroplatinate (K2PtCl6, Tianjin Kemiou Chemical Reagent Co., Ltd, AR, ≥99.5%), H2PtCl6.6H2O (Tianjin Kemiou Chemical Reagent Co., Ltd, AR, ≥99.5%), Eosin Y (EY, Sinopharm Chemical Reagent Co. Ltd., ≥85.0%), triethanolamine (TEOA, Xilong Chemical Co., Ltd., ≥98.0%), De-ionized water with a specific resistance of 18.2 MΩ·cm-2 was obtained by reverse osmosis followed by ion-exchange and filtration (RFD 250NB, Toyo Seisakusho Kaisha, Ltd., Japan).
1.2 Photochemical HER reactionSynthesis of catalysts, activity measurements of the photocatalytic H2 evolution and potassium transmutation to calcium were performed in a sealed Pyrex flask (150 mL) with a flat window (an efficient irradiation area of 10.2 cm2) and a silicone rubber septum for sampling. Reaction solutions were prepared as follows. The calculated amounts of potassium chloroplatinate, graphene oxides, Eosin Y and triethanolamine were mixed in water. For the blank test, hydrochloroplatinic acid was used instead of potassium chloroplatinate. For comparison, calculated amount of Ca(NO3)2·4H2O were added in the mixture solution in blank runs of visible irradiation. Typically, 1 mL graphene oxide suspensions (1 mg/mL), 70 mg Eosin Y, 1 mL K2PtCl solution (5 mg/mL) was added into above solution. After ultrasound vibration (25 kHz, 250 W) for 30 min, the mixture was degassed by bubbling Ar gas for 40 min. The photochemical HER reaction was carried out under irradiation of visible light ( > 420 nm), with continuous magnetic stirring.
1.3 CharacterizationThe amount of hydrogen evolution was measured using gas chromatograph (Aglient 6820, TCD, 13 x column, Ar carrier). The detection of D2 and He were carried out in a GC-MS (Aglient, 5975C, Triple-Axis Detector), a Quadrupole Mass Spectrometer (LC-D200M), and a Rare Gas Isotope Mass Spectrometry System (Nobleless SFT). The apparent quantum efficiency (AQE) was measured under the same photocatalytic reaction conditions with irradiation light through a band-pass filter (430, 460, 490, 520, or 550 nm). Photon flux of the incident light was determined using a Ray virtual radiation actinometer (FU 100, silicon ray detector, light spectrum, 400~700 nm; sensitivity, 10~50 μV μmol-1·m-2·s-1).The reactant mixture was irradiated for 30 min by a 300-W Xe lamp with a cutoff filter of 420 nm and a band pass filter for AQEs tests on the H2 production. The following equation was used to calculate the AQE.
| $\begin{array}{l} \quad AQEs = \\ \frac{{2{\rm{ \times the}}\;{\rm{number}}\;{\rm{of}}\;{\rm{evolved}}\;{\rm{hydrogen}}\;{\rm{molecules}}}}{{{\rm{the}}\;{\rm{number}}\;{\rm{of}}\;{\rm{incident}}\;{\rm{photons}}}}{\rm{ \times 100\% }} \end{array}$ |
Both colorimetric analysis and inductive coupled plasma emission spectrometry were used to measure the calcium concentration variation in the reaction mixture. Colorimetric analysis was carried out according to the reference method. Chlorophosphonazo-mA (CPA-mA) was used as a spectrophotometric reagent[27-28]. Molecular structure of CPA-MA is illustrated in Scheme 3[27-28]. Inductive Coupled Plasma Emission Spectrometer (ICP) measurement was conducted on an Agilent, 725-ES Inductive Coupled Plasma Emission Spectrometer (ICP-OES). The every detected calcium concentration datum was measured three times and the average value was presented.
2 Results and discussion 2.1 Colorimetric analysis and ICP measurementColorimetric analysis and ICP measurement were conducted to investigate the concentration variation of Ca2+ ions in the reaction mixture under irradiation. As the results shown in the Fig. 1, both colorimetric and ICP measurement showed similar increase tendency of Ca2+ ion concentration in the mixture during the photochemical HER reaction. In Fig. 1 (a), the transmittance of liquid samples at 650 nm decreased with the reaction time prolonging. The Fig. 1 (b) gives the measured Ca2+ ion concentrations by comparing standard curve and data in Fig. 1 (a). The calcium concentration in the reaction mixture increased from about 30 ppm to 42 ppm during the photochemical HER reaction which lasted for 90 min.

|
Figure 1 Colorimetric analysis results of photochemical HER system (adding K2PtCl6) (a) Transmittance of liquid samples at 650 nm over time (b) Ca2+ ions concentration over time |
To verify the colorimetric analysis, ICP measurement was conducted before (0 min) and after the photochemical HER reaction lasted for 60 min (λ ≥ 420 nm). As shown in Fig. 2, ICP measurements for every datum were repeated for three times and the average value was presented. Those ICP data obvious confirmed that the concentration increase of calcium in the reaction mixture after photochemical HER reaction for 60 min. If the initial concentration was around 12.5 ppm, the final calcium concentration increased to 16 ppm after 60 min reaction. Although there is deviation between colorimetric and ICP analysis, the increase tendency of calcium during the photocatalytic HER is similar.

|
Figure 2 ICP measurements of Ca2+ concentration before (0 min) and after photochemical HER reaction (60 min) (λ ≥ 420 nm) |
This is an exotic phenomenon that the increase of calcium concentration in the reaction mixture increa-sing during photochemical HER reaction. Given that the reaction system was conducted in well sealed container which could prevent any exogenous contaminate, there is almost no choice but to believe that some substance in the reactive mixture might be converted to Ca element. A feasible way is the potassium element transmutation into calcium via proton LENR, considering present bio-transmutation research mentioned in introduction section (39K + 1p = 40Ca+ ΔE). In this work, the potassium elements can come from the raw agent, K2PtCl6 in the photochemical HER system.
In order to prove above suggestion of this potential transmutation from potassium to calcium via proton LENR, comparative experiments were carried out by substituting K2PtCl6 with H2PtCl6 in the reaction mixture, thus there is no potassium element in the photochemical reaction system. Comparative experiments were repeated for two times, and their colorimetric analysis results are presented in Fig. 3. The results indicated that almost no variation of calcium concentration during the photocatalytic HER. Those results provide evidence that the increase of calcium concentration in the reaction mixture is closely related to the presence of potassium, namely, indicating elemental transmutation from potassium to calcium.

|
Figure 3 Colorimetric analysis results of photochemical HER system (substituting K2PtCl6 with H2PtCl6·6H2O) (a) Transmittance at 650 nm over time (b) Ca2+ ions concentration over time |
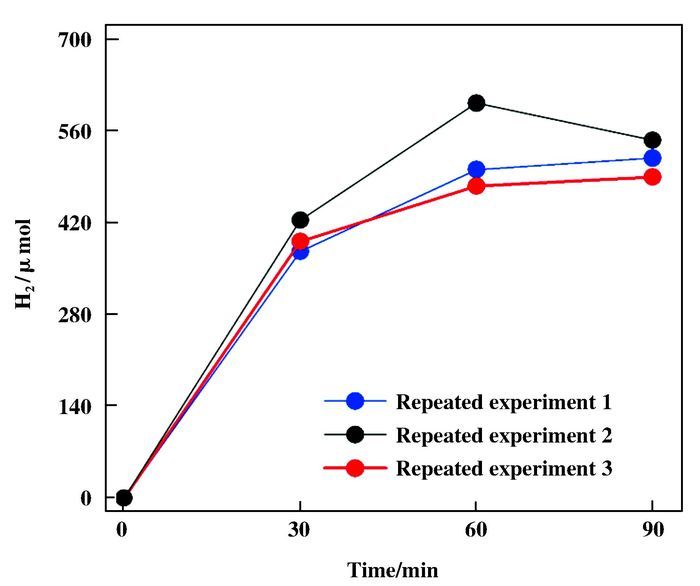
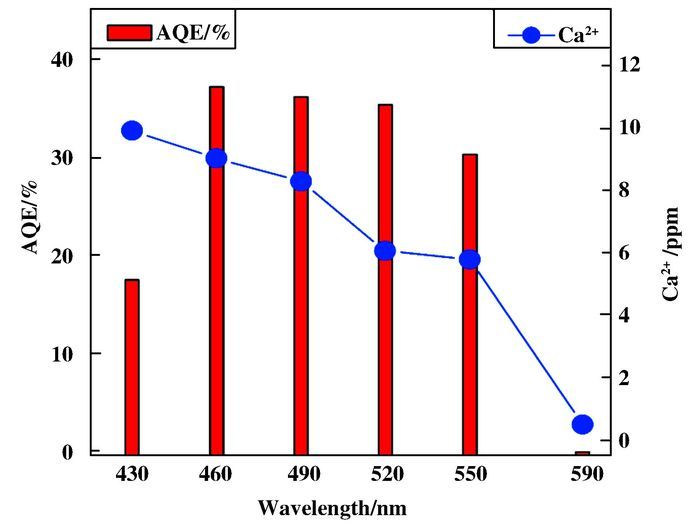
To understand the correlation of potassium transmutation with hydrogen formation during photocatalytic HER, we investigate more details of the photochemical HER reaction process. Hydrogen evolution tests were conducted the three repeated experiments and the corresponding results were presented in Fig. 4. Clearly, hydrogen evolution increased gradually during the 90 min of photochemical HER reaction. The hydrogen was continuously produced when the mixture was irradiated by visible light. The results of apparent quantum efficiency (AQE) for hydrogen generation revealed that proton reduction (or H2 evolution) was highly depen-dent on the input light wavelength. AQE for hydrogen generation was 18% at 420 nm, and increased to 38% when the irradiation wavelength was 460 nm, then remained around 35% between 490~520 nm. When the irradiation wavelength was 590 nm, the AQE for hydrogen generation was less then 1%. Accordingly, the variation of calcium concentration in the mixture decreased when AQE value lowered down (Fig. 5).
|
Figure 4 H2 production of photochemical HER system (adding K2PtCl6) over time (λ ≥ 420 nm) |
|
Figure 5 Absolute quantum efficiency (AQE) and Ca2+ concentration variation of photochemical HER system (adding K2PtCl6) over time (λ ≥ 420 nm) |
We also observed trace amount of 3He and 4He formation during phtotocatalytic HER, and the results were given in Fig. 6. Although it is believed that helium can be generated from hydrogen in nuclear reaction under very high temperature and high pressure, however, we previously observed the helium formation during phtotocatalytic HER under normal conditions[25-26]. The formation of helium in photocatalytic HER reaction indicate that there is possibility of occurrence of low energy nuclear reaction (LENR) during hydrogen formation. We previously proposed that the formation of helium might be related to the existence of protons weakly bound by electrons (such as negative hydrogen, H-). Actually, Fleischmann and Pons reported that LENR phenomenon in mild electrochemical reaction conditions, and they guessed it correlated to the fusion of heavy hydrogen atoms. Our group recently reported the generation of deuterium and helium from proton LENR in photochemical reaction system, and suggested that the intermediate complex substance was composed of protons with weakly bound electrons (such as negative hydrogen, H-)[25-26]. In this work, discovering trace amount of 3He and 4He showed the evidences of negative hydrogen (H-) existing in the photochemical HER reaction system and its role in LENR.

|
Figure 6 Mass spectra of gas evolved from Repeated experiment 1 |
Those above experimental data make it reasonable to find some kinds of relationship between Ca2+ concentration increasing with H2 evolution and 3He/4He yielding, in other words, calcium element formation is accompanied with the process of proton reducing and the process of potassium transmutation. Those discoveries may provide another evidence to uncover the mysteries of elemental transmutations in "mild" reactive systems from a new point of view. Actually, photochemical HER systems have some unique characteristics. They provide hydrogen atoms with a multi-electron interactions and reductive environment (Scheme 4)[21-24], thus facilitating the yield of H- ions under photon irradiation[25-26]. As H- is active in reaction system, it can not only combine with H+ to form deuterium and helium atoms (H+ + H- → 2D → He)[25-26], but are promising to facilitate the transmutation from potassium to calcium.
2.3 Plausible mechanism of transmutation process in photochemical HER systemBased on obtained data, a plausible transmutation process in photochemical HER reaction might be as follow: supposing a H atom was produced via negative hydrogen ion (H-) throwing out an electron (H--e = H), a K+ ion can capture a proton or a negative hydrogen and then release two electron to transmute into Ca2+ ion via β decay. These processes could be described as:
K+ + H+→ Ca2+ → ΔE (Formula 1)
or K++ H- → Ca2+→ 21 0 e + ΔE (Formula 2)
Herein, positive hydrogen (H+) is equal to a proton, negative hydrogen (H-) is a complex substance composed of a hydrogen atom which weakly binds an electron. Formula 2 is more reasonable than formula 1 because the strong electrostatic repulsion will keep K+ and H+ separated. Here we did not consider the contributions of neutrino and anti-neutrino but we believed they might involve β decay in electron release. As for the mass difference before and after reaction in formula 2, there should corresponds some energy variation during the reaction. According to the mass-energy equation, E = Δmc2, the mass difference may correlate energy releasing or new matter formation, for example, some quarks.
K+ + H- = Ca2+ + 2e- + Δm (meson or s -1/3+ u +2/3+ d -1/3) (Formula 3)
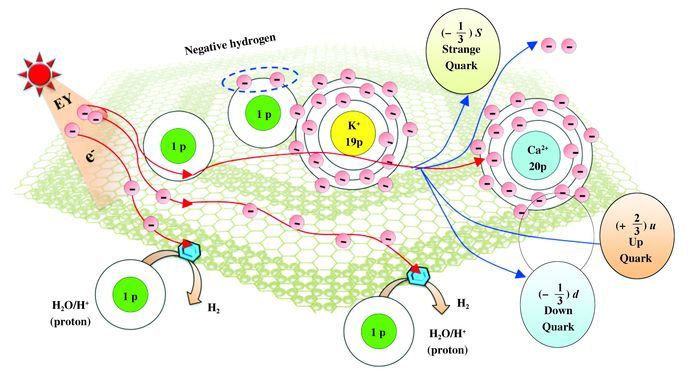
Occurrence of transmutation process could mainly be attributed to the uniqueness of photochemical HER system, especially the contribution of negative hydrogen (H-) to lowering the energy barrier of LENR, as illustrated in Scheme 5. When a H- particle approaches the nucleus of a K+ ion, strong electronic interaction between the negative hydrogen and the nucleus of K+ ion might lead to such a LENR of potassium transmutation to calcium because catalyst system provided an suitable reaction and energy environment with multi-electron and nuclei interaction (coupling).
|
Scheme 5 Proposed process of potassium-Calcium transmutation in photochemical HER system |
We can suppose that potassium-calcium transmutation is beneficial for maintaining human body health. Calcium is an important element for human body, like constituting the bones and teeth and helping heart and other muscles do their work normally[27-28]. Low calcium intake can lead to fragile bones, high blood pressure, and certain types of cancer. Furthermore, recent studies have shown that calcium may reduce the risk of colon cancer, and have potential of preventing breast and pancreas cancers when combined with vitamin D. Actually, it is found that negative hydrogen can act as antioxidant by selectively reducing cytotoxic oxygen radicals[30]. It could be suspected that there might be hydrogen which existing as negative hydrogen in human body, for H- ions are more solvable in body fluids compared with H2 molecule. Those H- ions could faci-litate elements transmutation in organism body.
The reported data and proposed mechanism might be a potential answer to a long-lastingly unsettled question, that what environment can induce"mild" element transmutation, for example, the transmutation in biological bodies and bionics reactive system, typically, energy release and new matter formation during such a LENR. It can also help to answer the question that why the chickens can produce so many eggs while they do not take in enough calcium, and how can organisms take in some special elements even they grow in isolated environments. In addition, the mitochondrion in cells might capture the energy released from transmutation, accumulate those energies, and release them slowly for organism metabolism.
3 ConclusionIn conclusion, this work finds some exotic and interesting results about potassium transmutation to calcium in photochemical HER system driven by visible light irradiation.The concentration increase of calcium element in the mixture dispersion is accompanied with the process of proton reduction (H2 evolution) and proton fusion which yields 3He and 4He. Those results indicate that some of calcium elements in nature might originate from potassium-calcium transmutation through low energy nuclear reaction (LENR) under very mild conditions, which might be related to the yield of negative hydrogen (H-) during photocatalytic hydrogen generation. We realize those discoveries may raise some questions and arguments.
Acknowledgement: This work has been supported by the National Natural Science Foundation of China (Grant No. 21433007 and 21673262) and the 973 Program of Department of Sciences and Technology China (Grant No. 2013CB632404).| [1] | Schaeffer O A, Zähringer J. Solar flare helium in satellite materials[J]. Phys Rev Lett, 1962, 8(10): 389. DOI:10.1103/PhysRevLett.8.389 |
| [2] | Badash L. Radium, Radioactivity and the popularity of scientific discovery[J]. Proc Am Philos Soc, 1978, 122(3): 145–154. |
| [3] | Howorth M. Pioneer research on the atom: Rutherford and soddy in a glorious chapter of science; the life story of Frederick Soddy[M]. New World Publications, 1958. |
| [4] | Trenn T J. The Self-Splitting Atom: The history of the rutherford-soddy collaboration[M]. London: Taylor & Francis, 1977(p. 42, 58-60, 111-17). |
| [5] | Cockcroft J D, Walton E T S. Artificial Production of Fast Protons (Reprinted from Nature, February 13, 1932)[J]. Nature, 1969, 224: 463. DOI:10.1038/224463a0 |
| [6] | Henderson M C. The disintegration of lithium by protons of high energy[J]. Phys Rev, 1933, 43(2): 98–102. DOI:10.1103/PhysRev.43.98 |
| [7] | Paneth F A, Günyher P L. Chemical detection of artificial transmutation of elements[J]. Nature, 1933, 131: 652–653. |
| [8] | Bush R P. Recovery of platinum group metals from high level radioactive waste[J]. Plat Met Rev, 1991, 35(4): 202–208. |
| [9] | Guerra F, MLeone M, Robotti N. The discovery of artificial radioactivity[J]. Phys Pers, 2012, 14(1): 33–58. DOI:10.1007/s00016-011-0064-7 |
| [10] | Kervran C L. Biological transmutations[M] (revised and edited by H. Rosenauer and E. Rosenauer, Crosby Lockwood, London 1972, reprinted by Beekman, New York, 1980, reprinted 1998). |
| [11] | Biberian J P. Biological transmutations:Historical perspective[J]. J Cond Mat Nucl Sci, 2012, 7: 11–25. |
| [12] | Biberian J P. Biological transmutations[J]. .Curr. Sci., 2015, 108(4): 633–635. |
| [13] |
a. Kervran C L. Biological transmutations and modern physics, Maloine S. A. Publisher, Paris, ISBN 2-224-00831-7, 1982. b. Kervran C L. Biological transmutations and modern physics[M] (http://www.rexresearch.com/kervran/kervran.htm). |
| [14] | Emad Y M. Nuclear transmutation and cancer in the biological cell[J]. Int J Biochem Biophys, 2013, 1: 1–8. |
| [15] | Timashev S F. Initiating nuclear-chemical transformations in native systems:Phenomenology[J]. Russ J Phys Chem A, 2016, 90(10): 2089–2095. DOI:10.1134/S0036024416100253 |
| [16] | Vysotskii V I, Adamenkob S V, Vysotskyy M V. Acce-leration of low energy nuclear reactions by formation of correlated states of interacting particles in dynamical systems[J]. Ann Nucl Energy, 2013, 62: 618–625. DOI:10.1016/j.anucene.2013.02.021 |
| [17] | Zeitlin C, Hassler D M, Cucinotta F A, et al. Measurements of energetic particle radiation in transit to mars on the mars science laboratory[J]. Science, 2013, 340(6136): 1080–1084. DOI:10.1126/science.1235989 |
| [18] | Vezzoli G C. Beta decay and quark-antiquark non-parity in collision-induced gravity[J]. Prog in Phys, 2009, 2: 72–76. |
| [19] | Nakamura S X, Kamano H, Hayato Y, et al. Towards a unified model of neutrino-nucleus reactions for neutrino oscillation experiments[J]. Rep on Prog in Phys Phys Soc, 2017, 80(5): 056301. DOI:10.1088/1361-6633/aa5e6c |
| [20] | Bilenky S M, Hošek J. Glashow-Weinberg-Salam theory of electroweak interactions and the neutral currents[J]. Phys Rep, 1982, 90(2): 73–157. DOI:10.1016/0370-1573(82)90016-3 |
| [21] | Tian B, Li Z, Zhen W L, et al. Uniformly sized (112) facet Co2P on graphene for highly effective photocatalytic hydrogen evolution[J]. J Phys Chem C, 2016, 120(12): 6409–6415. DOI:10.1021/acs.jpcc.6b00680 |
| [22] | Zhang X Q, Lu G X. The spin-orbit coupling induced spin flip and its role in the enhancement of the photocatalytic hydrogen evolution over iodinated graphene oxide[J]. Carbon, 2016, 108: 215–224. DOI:10.1016/j.carbon.2016.07.022 |
| [23] | Zhang W Y, Kong C, Lu G X. Super-paramagnetic nano-Fe3O4/graphene for visible-light-driven hydrogen evolution[J]. Chem Commun, 2015, 51(50): 10158–10161. DOI:10.1039/C5CC01925G |
| [24] | Kong C, Min S X, Lu G X. Dye-sensitized cobalt catalysts for high efficient visible light hydrogen evolution[J]. Int J Hydrogen Energy, 2014, 39(10): 4836–4844. DOI:10.1016/j.ijhydene.2014.01.089 |
| [25] | Lu Gong-xuan(吕功煊), Tian Bin(田彬). Formation of deuterium and helium during photocatalytic hydrogen generation from water catalyzed by Pt-graphene sensitized with Br-dye under visible light irradiation(溴染料敏化担载Pt石墨烯催化可见光制氢、氘和氦)[J]. J Mol Catal (China)(分子催化), 2017, 31(2): 101–104. |
| [26] | Lu Gong-xuan(吕功煊), Zhen Wen-long(甄文龙). Formation of helium-3 and helium-4 during photocatalytic hydrogen generation over cadmiun sulfide under visible light irradiation(半导体CdS悬浮体系中可见光催化产氢同时生成氦-3和氦-4)[J]. J Mol Catal (China)(分子催化), 2017, 31(4): 299–304. |
| [27] | Chen Y, Gu W J, Pan H H, et al. Stabilizing amorphous calcium phosphate phase by citrate adsorption[J]. CrystEngComm, 2014, 16(10): 1864–1867. DOI:10.1039/C3CE42274G |
| [28] | Qiu X C, Zhang Y S, Zhu Y Q. Spectrophotometric determination of exchangeable calcium in soils by chlorophosphonazo-mA[J]. Analyst, 1983, 108(1287): 754–757. DOI:10.1039/an9830800754 |
| [27] | Boron W, Barrett P. The parathyroid glands and vitamin D[M]. Medical Physiology (2nd Ed), Published by Elsevier/Saunders, ISBN 1-4160-2328-3, 2012. |
| [28] | Pravina P, Sayaji D, Avinash M. Calcium and its Role in Human Body[J]. Int J Res in Pharm Biomed Sci, 2013, 4(2): 2229–3701. |
| [29] | Dougherty D P, Wright G A, Yew A C. Computational model of the cAMP-mediated sensory response and calcium-dependent adaptation in vertebrate olfactory receptor neurons[J]. Proc National Acad Sci, 2005, 102(30): 10415–10420. DOI:10.1073/pnas.0504099102 |
| [30] | Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals[J]. Nat Med, 2007, 13(6): 688–694. DOI:10.1038/nm1577 |
 2017, Vol. 31
2017, Vol. 31