2. University of Chinese Academy of Sciences, Beijing 100049, China
2. 中国科学院大学, 北京 100049
Supported ruthenium catalysts exhibit much higher activity at lower temperatures and pressures than the conventional fused iron catalysts in the synthesis reaction of ammonia[1-2]. As a result, extensive efforts[3-6] had been focused on the development of the effective Ru-based catalysts. Especially, the focal point is the effect of the support on the Ru nanoparticles for dissociative adsorption of N2 molecular[7-8]. Further, the interface between the Ru nanoparticles and the support plays an important role in the ammonia synthesis reaction such as the interface composition, structure and electron properties. For example, with a graphitic carbon materials as the support[9-11, 30-31], the enhancing catalytic activity was ascribed to its high electron conductivity of graphite structure and its electron-donating composition (the electronic promoter and the electron-rich element). In addition, the basicity of support has been proved that it was relative with the rate of ammonia synthesis due to the basic support can donate electrons to Ru atoms[12]. However, these supports consisting of particles are so complex solid systems with ill-defined surface species that it is difficult to control the interface composition, structure and electron properties.
The rare-earth oxides (CeO2, La2O3 and Pr2O5 etc.) with reversible redox properties and abundant of oxygen vacancies have been proved an effective support or the promoter for the ammonia synthesis reaction[13-15]. In our previous work[16], three ceria nanocrystals with different morphologies (nanocubes, nanorods, and nanoparticles) were synthesized and used as the supports to evaluate the support morphology-dependent ammonia synthesis activity. The catalytic performance of Ru/CeO2 catalysts is correlated to the exposed crystal planes and surface composition and electronic structure of the CeO2 support with diverse morphologies. Similarly, the morphology of CeO2 support was found to be a key factor determining the catalytic performance of Co catalyst for ammonia synthesis[17]. Therefore, it was high desirable to control the morphology of the support and simultaneously modulate the interface properties between the support and Ru particles.
Herein, we employed the different morphologies of Pr-doped CeO2 as supports to investigate the effect of the morphology and the interface composition on the ammonia synthesis activity. The physical and chemical properties of the catalyst were investigated by TEM, XRD, UV-vis, XPS, and TPR analyses. The catalysts exhibit significant support-morphology-dependent catalytic performances for the ammonia synthesis reaction, which could be correlated to the exposed crystal planes and surface composition of the Pr-doped CeO2 nanocrystals.
1 Experimental section 1.1 ChemicalsAll chemicals were of analytical grade and used directly without any further purification. They were purchased from Aladdin Industrial Corporation in China. The purity of nitrogen and hydrogen was 99.999%. Deionized water with a resistivity of 18.25 MΩ·cm was used in all the reactions.
1.2 Preparation of Ce0.8Pr0.2O2 supportsThe Ce0.8Pr0.2O2 nanorods and nanocubes supports were prepared by a hydrothermal method. In a typical experiment, 5.568 g of Ce(NO3)3·6H2O, 1.392 g of Pr(NO3)3·6H2O and an appropriate amount of NaOH were dissolved in 40 mL of deionized water. Subsequently, the solution was directly transferred to a 50 mL Teflon lined stainless steel autoclave without stirring, and maintained at an appropriate temperature (100 ℃ for nanorods donated as r-Ce0.8Pr0.2O2, 180 ℃ for nanocubes donated as c-Ce0.8Pr0.2O2) for 24 h. After the hydrothermal treatment, the precipitates were washed with deionized water until the pH=7, and then dried at 100 ℃ for 12 h, followed by calcination at 450 ℃ for 4 h in air at a heating rate of 3 ℃/min.
1.3 Preparation of Ru/Ce0.8Pr0.2O2 catalystsThe Ru/Ce0.8Pr0.2O2 catalysts were prepared using the impregnation method. 1 g of Ce0.8Pr0.2O2 powders with different morphologies were stirred with a solution of ruthenium carbonyl (Ru3(CO)12) in 40 mL of THF at room temperature for 12 h by magnetic stirring. Then the solvent was removed under reduced pressure at 40 ℃. The theoretical loading amount of ruthenium was 4%. The palm red powder was calcined at 300 ℃ for 3 h in a hydrogen atmosphere at a heating rate of 3 ℃/min, followed by cooling down to room temperature in a hydrogen atmosphere.
1.4 Measurements of catalytic activityIn the ammonia synthesis reaction, 0.2 g catalyst was used for each experiment. The catalyst was diluted with quartz sand to 0.6 mL (stainless steel microreactor (d=6 mm)). The catalysts were stabilized under diffe- rent temperatures for 2 h (reactor conditions: 1 MPa, 60 mL/min, and H2:N2= 3). When the ammonia synthesis reaction reached a steady state, data were obtained. The ammonia synthesis rates in the effluent were determined via a chemical titration method with a known amount of H2SO4 (Congo red as the indicator).
1.5 Characterization of the catalystsThe crystalline structures of the samples were analyzed via X-ray powder diffraction (XRD) (X'pert, PANalytical, Dutch) using Cu Kα radiation (d=1.540 50). Transmission electron microscope (TEM) experiments were conducted using a JEM-2010 with an accelerating voltage of 200 kV. The element compositions were detected using an X-ray photoelectron spectrometer (XPS, ESCALAB 250Xi). The electron bin- ding energy scale of all the spectra was calibrated using C 1s at 284.8 eV. Temperature programmed reduction (TPR) profiles of the samples were generated using a TP-5080 catalyst characterization instrument with a TCD detector. Before the H2-TPR test, the samples (50 mg) were pretreated with an Ar flow (27 mL/min) at 300 ℃ for one hour and were then cooled down to 25 ℃. The test was performed by heating the samples (50 mg) in a H2/Ar (H2, 10%) mixture flow (30 mL/min) at a linear heating ramp of 10 ℃/min from 25 to 800 ℃.
2 Results and discussionThe morphology of the synthesized c-Ce0.8Pr0.2O2 and r-Ce0.8Pr0.2O2 samples was investigated by TEM and HRTEM in Fig. 1. Fig. 1A shows the uniform nanocube morphology of c-Ce0.8Pr0.2O2, which the side size of nanocubes is about 300 nm. And Fig. 1C shows the nanorod morphology of r-Ce0.8Pr0.2O2, which has a length in the range of 100~200 nm. As the previous report[18], the nanocubes of c-Ce0.8Pr0.2O2 selectively exposes the (100) planes with an interplanar spacing of 0.286 nm (Fig. 1B). The nanorods of r-Ce0.8Pr0.2O2 mainly expose the (110) and (100) planes. An interplanar spacing of 0.193 nm in Fig. 1D is attributed to the (110) plane of r-Ce0.8Pr0.2O2 and it can be noted that the r-Ce0.8Pr0.2O2 grow along (110) and show 1D growth.

|
Figure 1 TEM images of the as-prepared Ce0.8Pr0.2O2 nanocrystals: (A, B) nanocubes (c- Ce0.8Pr0.2O2), (C, D) nanorods (r-Ce0.8Pr0.2O2); TEM images of Ru/Ce0.8Pr0.2O2:E) nanorod catalyst (Ru/r-Ce0.8Pr0.2O2); F)nanocube catalyst(Ru/c-Ce0.8Pr0.2O2) |
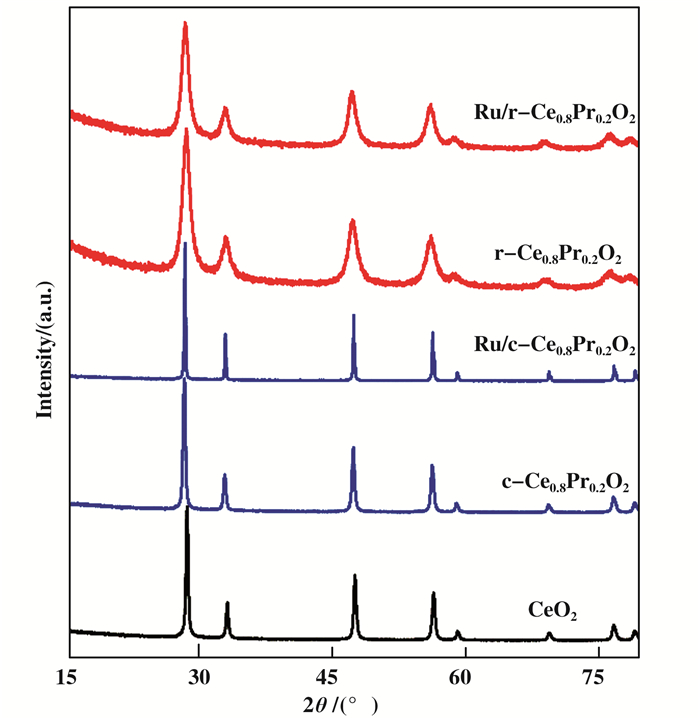
The crystal structures of the as-synthesized Ce0.8Pr0.2O2 and Ru/Ce0.8Pr0.2O2 are investigated by XRD techniques and shown in Fig. 2. The diffraction peaks at 28.4°, 32.9°, 47.4°, 56.2° and 58.9° assigned to the (111), (200), (220), (311) and (222) planes are indexed to the cubic fluorite structure of CeO2 crystals (JCPDS No. 34-0394). The peaks indexed to praseodymium oxide and Ru metal are not detected in all the samples, indicating that Pr was successfully incorporated into the ceria lattice structure and the Ru species are highly dispersed in the catalysts. Moreover, the elemental mapping of different morphology Ru/Ce0.8Pr0.2O2 catalysts disclosed that the Ru nanoparticles are dispersed on the support (Fig.S1 and Fig.S2). Beside, the TEM image of the catalysts Ru/c-Ce0.8Pr0.2O2 and Ru/r-Ce0.8Pr0.2O2 also showed the Ru was dispersed on the support (Fig. 1E), F)). The peaks of c-Ce0.8Pr0.2O2 were sharper than that of r-Ce0.8Pr0.2O2, which indicated the degree of crystallinity of c-Ce0.8Pr0.2O2 was much higher than r-Ce0.8Pr0.2O2. As shown in Fig. 1B, D, the interplannar spacing of the Pr-doped CeO2 was 0.193 nm (110) and 0.286 nm (100), while that of pure CeO2 was 0.191 nm (110) and 0.27 nm (100). Therefore, We can rationally speculate that Pr was successfully incorporated into the ceria lattice structure to form fluorite-like solid solution and this increase is mainly due to the replace-ment of Ce4+(0.087 nm) by bigger radius of Pr3+ (0.099 nm), which is in accordance with the XPS results.
|
Figure 2 The XRD patterns for the different morphologies of Ce0.8Pr0.2O2 and the corresponding supported Ru catalysts |
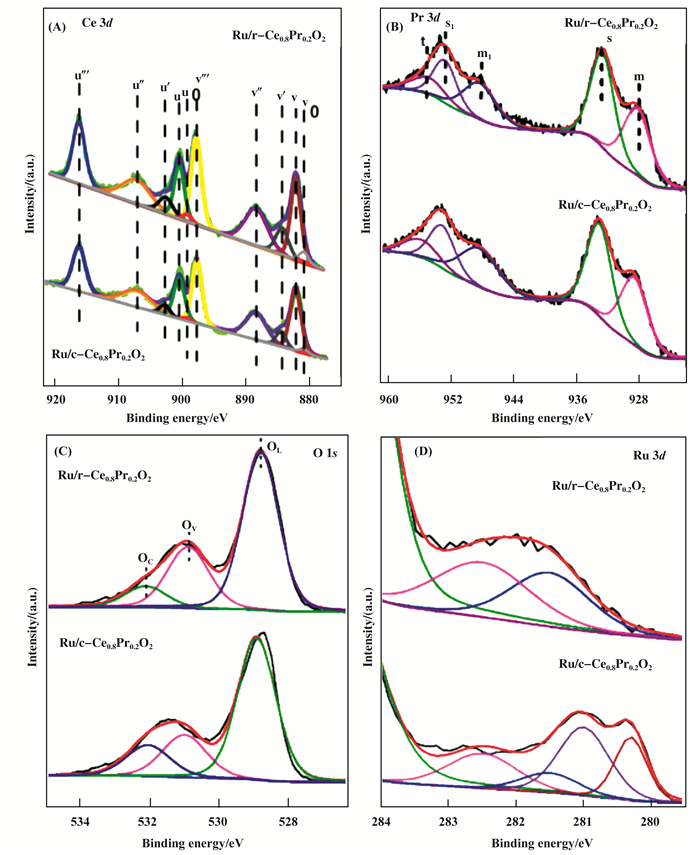
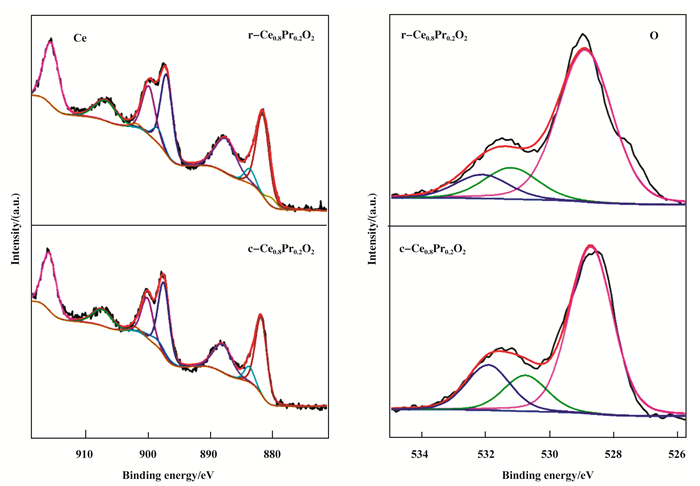
Recently, theoretical calculations[19-21] indicate that the different crystal planes of Ce0.8Pr0.2O2 could strongly affect the oxygen vacancy formation energy and the interaction with surface molecules. As such, the detailed surface electronic states of the catalyst were investigated by the XPS spectra. Fig. 3 showed the high-resolution XPS spectra of Ce 3d, Pr 3d, Ru 3d, and O 1s for Ru/r-Ce0.8Pr0.2O2 and Ru/c-Ce0.8Pr0.2O2. Fig. 4 showed the high-resolution XPS spectra of Ce 3d, O 1s for r-Ce0.8Pr0.2O2 and c-Ce0.8Pr0.2O2. The XPS spectra of Ce 3d are fitted into ten components[22]: the state of Ce4+ species are labeled as u″′ (916.2 eV), u″ (907 eV), u (900 eV) and v″′ (897.7 eV), v″ (888.4 eV), v (882.2 eV), whereas the state of Ce3+ are labeled as u′ (902.7 eV), u0 (899.2 eV), v′ (884.45 eV), and v0 (880.9 eV). The relative element percentages of Ru/Ce0.8Pr0.2O2 are obtained from the area ratio of the peaks (Table 1). The support r-Ce0.8Pr0.2O2 consist of 91.5% Ce4+ and 8.5% Ce3+. After loading Ru, the Ce3+ of the catalyst Ru/r-Ce0.8-Pr0.2O2 ((110) and (100) facet) increased to 13.3%, while the Ce4+ decreased to 86.7%. However, the Ce3+ species of support c-Ce0.8Pr0.2O2 decreased to 8.1% and the amount of Ce4+ increased 91.9%, besides, after loading Ru, the amount of Ce3+ species decreased to 8.6% and the amount of Ce4+ increased to 91.4% when the support only exposed the (100) facet. In the previous report, the concentration of Ce3+ was related to that of oxygen vacancies due to excess electrons left behind by the removal of neutral oxygen localized not only on the oxygen vacancy but also on the Ce 4f state. The O 1s spectra could be fitted with three peaks[23] at 528.9 eV (OL), 531.2 eV (Ov) and 532.1 eV (Oc), which can be attributed to lattice oxygen bound to metal cations, O2- in oxygen-deficient regions, and the chemisorbed and dissociated oxygen species, respectively. As a consequence, the number of surface oxygen vacancies could be roughly estimated based on the ratio of Ov/OL. As shown in Table 1, the r-Ce0.8Pr0.2O2 and the cooresponding catalyst Ru/r-Ce0.8Pr0.2O2 catalyst has more oxygen vacancies than that of the c-Ce0.8Pr0.2O2 and the cooresponding catalyst Ru/c-Ce0.8Pr0.2O2 catalyst. In addition, the Pr ions dominantly possess the +3 valence state[24], which can be classified into two different states: (a) Pr 3d5/2 at lower binding energy (928~933 eV), in which two peaks are denotated as m and s, (b) Pr 3d3/2 at higher binding energy (947~953 eV). While an additional weak peak labeled by t appears at 955.4 eV as a marker of Pr4+ oxidation state. Therefore, the exposed (100) and (110) planes of the Ce0.8Pr0.2O2 have various types of surface composition.
|
Figure 3 The High resolution XPS spectra of Ce 3d, Pr 3d, O 1s, and Ru 3d |
|
Figure 4 The High resolution XPS spectra of the as-prepared Ce0.8Pr0.2O2 nanocrystals:Ce 3d, O 2p |
| Table 1 Atomic ratio of different morphologies catalysts |
Different exposed planes of the Ce0.8Pr0.2O2 support have various types of surface geometries or electronic structures and compositions, which could affect the chemical state of the active metal when being loa- ded on the different crystal planes. The Ru 3d XPS spectra of the catalysts(Fig. 3D) can be observed a doublet corresponding to Ru 3d5/2 and Ru 3d3/2. The binding energy at 280.3 eV is the characteristic of metallic ruthenium (Ru0)[25]. The shift in the peaks for Ru 3d5/2 to higher values[26] (281.1 and 282.5 eV) can be attributed to RuO2 and RuO3. The peak at 281.6 eV can be ascribed to RuOx, which most likely exists at the interface between Ru nanoparticles and Ce0.8Pr0.2O2 support[27]. Obviously, the exposed (110) and (100) planes of Ce0.8Pr0.2O2 support affect the chemical state of Ru species. Compared with the Ru/r-Ce0.8Pr0.2O2 catalyst, it have metallic ruthenium (Ru0) and lower valence RuOx in the Ru/c-Ce0.8Pr0.2O2. Thus, it can be concluded that the exposed (100) and (110) planes of the Ce0.8Pr0.2O2 have various types of surface electronic structures.
H2-TPR was employed to investigate the reducibility of the Ce0.8Pr0.2O2 supports and Ru/Ce0.8Pr0.2O2 catalysts (Fig. 5). The c-Ce0.8Pr0.2O2 and r-Ce0.8Pr0.2O2 supports exhibit strong reduction peaks at 467 ℃ and 400~510 ℃, respectively, which could be attributed to the reduction of Ce0.8Pr0.2O2 surface oxygen. However, the surface oxygen reduction of c-Ce0.8Pr0.2O2 was much more difficult than that of r-Ce0.8Pr0.2O2. These results imply that the oxygen in (110) plane formed the stronger chemical bonds to Ce or Pr than that in (110) plane, which is in accordance with oxygen vacancy formation energy on the different crystal planes of Ce0.8Pr0.2O2. After loading Ru on the support, the surface oxygen reduction of Ce0.8Pr0.2O2 clearly shifted to lower temperatures. Given that the samples were pretreated with Ar for 1h, the reduction peaks at T < 150 ℃ should be caused by the weakening of the Ce-O and Pr-O bond by the strongly bound Ru species. Further comparing the H2 consumption amount of surface oxygen in the Ce0.8Pr0.2O2 and Ru/Ce0.8Pr0.2O2, the H2 consumption amount of Ru/c-Ce0.8Pr0.2O2 and Ru/r-Ce0.8Pr0.2O2 are 2.7 times and 1.5 times larger than that in corresponding support, respectively. These indicated the loading of Ru can lead to a results of formation the metal hydride CeH2+x and PrH2+x. Moreover, the earlier investigation[28-29] has reported that CeH2+x hydride can easily form on the interface of Ce-Ru. Thus, we can rationally deduce that the metal hydride formed the different composition and electron properties on the (110) and (100) planes of the catalyst.

|
Figure 5 H2-TPR profiles for the different morphologies Ce0.8Pr0.2O2 and the corresponding supported Ru catalysts |
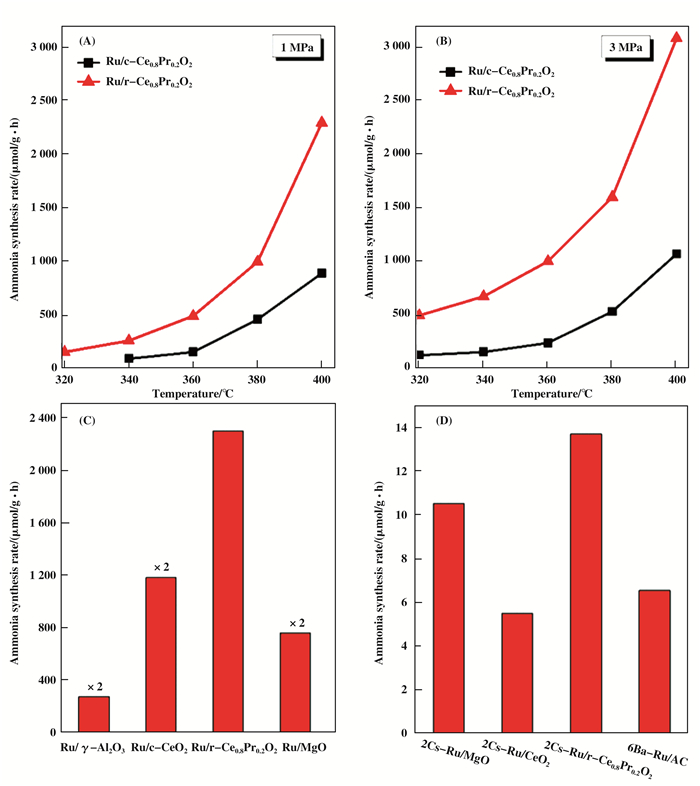
The catalytic activities of the two catalysts for ammonia synthesis are illustrated in Fig. 6. It can be noted that the reaction rate of the Ru//r-Ce0.8Pr0.2O2 catalyst was 2 297.1 μmol/(g·h), which was 2.6 times higher than that of Ru/c-Ce0.8Pr0.2O2(887.6 μmol/(g·h)) at a reaction temperature of 400 ℃ and the pressure of 1 MPa (Fig. 5A). With the increasing of the pressure to 3 MPa, the activity of Ru/Ce0.8Pr0.2O2 was also increasing such as Ru/r-Ce0.8Pr0.2O2 (3 089.6 μmol/(g·h)) and Ru/c-Ce0.8Pr0.2O2 (1 067.6 μmol/(g·h)) (Fig. 5B). These results indicate that the planes of the Ce0.8Pr0.2O2 supports has a significant effect on the ammonia synthesis activity of the catalysts. In the previous report, it is widely accepted that the interface between Ru and the support plays a key role in ammonia synthesis such as the basicity, the acidity, rich-electron and electron conductibility. As such, several catalysts were used to contrast the ammonia synthesis activity. The catalytic activity of Ru/CeO2 catalyst is higher than that of Ru/γ-Al2O3 and Ru/MgO, which could be ascribed to the formation of CeH2+x hydride due to the H- ions can transfer electrons to Ru and reversibly store and release hydrogen. Besides, the activity of Ru/r-Ce0.8Pr0.2O2 is higher than that of Ru/CeO2. The effect of the promoters on the ammonia synthesis activity of the catalysts were also investigated. The reaction rates of 6Ba-Ru/AC and 2Cs-Ru/MgO catalysts are 6 540 μmol/(g·h) and 10 530 μmol/(g·h), respectively. The former is used for commercial ammonia production and the latter is one of the most active Ru catalysts for ammonia synthesis. However, introducing the Cs promoter in the Cs-Ru/Ce0.8Pr0.2O2 catalyst, the reaction rate of 2Cs-Ru/r-Ce0.8Pr0.2O2 and 2Cs-Ru/c-Ce0.8Pr0.2O2 are clearly increased to 13 698.6 μmol/(g·h) and 7 667.7 μmol/(g·h), respectively. These results suggest that the effect of the plane of Ce0.8Pr0.2O2 support on the catalytic performance of Ru/Ce0.8Pr0.2O2 and 2Cs-Ru/Ce0.8Pr0.2O2 catalysts could be correlated to the Pr doping into CeO2 altered the interface properties (composition and electron structure) between the Ru nanoparticles and the Ce0.8Pr0.2O2 support.
|
Figure 6 A, B) Ammonia synthesis plot of Ru/Ce0.8Pr0.2O2 in the temperature range of 320~400 ℃, reaction conditions: N2:H2= 1:3, 1 MPa and 3 MPa; C) The catalyst activity comparison over different 4% Ru-loaded catalyst at 400 ℃ and 1 MPa on ammonia synthesis; D) Activity comparison of four different catalysts with the promoters. Reaction conditions: 400 ℃; N2:H2= 1:3; 1 MPa |
We have synthesized two different morphologies of Ce0.8Pr0.2O2 supports (nanocubes and nanorods), which dominantly exposed the (100) and (110). The XPS spectra verify that the surface composition and electronic structure of various facets are different and further affect the chemical state of Ru species. The effect of the plane of Ce0.8Pr0.2O2 support on the catalytic performance of Ru/Ce0.8Pr0.2O2 and 2Cs-Ru/Ce0.8Pr0.2O2 catalysts was correlated to the Pr doping into CeO2 altered the interface properties (composition and electron structure) between the Ru nanoparticles and the Ce0.8Pr0.2O2 support.
Acknowledgements: This research was financially supported by the National Natural Science Foundation of China (Grant No. 21403257).| [1] | Schlögl R. Catalytic Synthesis of Ammonia-A "Never-Ending Story"[J]. Angew Chem Int Ed, 2003, 42(18): 2004–2008. DOI:10.1002/anie.200301553 |
| [2] | Kitano M, Inoue Y, Yamazaki Y, et al. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store[J]. Nat Chem, 2012, 4(11): 934–940. DOI:10.1038/nchem.1476 |
| [3] | Saadatjou N, Jafari A, Sahebdelfar S. Ruthenium nanocatalysts for ammonia synthesis:A review[J]. Chem Eng Commun, 2015, 202(4): 420–448. DOI:10.1080/00986445.2014.923995 |
| [4] | Li Y, Pan C, Han W, et al. An efficient route for the preparation of activated carbon supported ruthenium catalysts with high performance for ammonia synthesis[J]. Catal Today, 2011, 174(1): 97–105. DOI:10.1016/j.cattod.2011.01.053 |
| [5] | McClaine B C, Siporin S E, Davis R J. X-ray and IR spectroscopy of barium-promoted, zeolite-supported ruthenium catalysts for ammonia synthesis[J]. J Phy Chem B, 2001, 105(31): 7525–7532. DOI:10.1021/jp011027n |
| [6] | Zhou C H, Zhu Y F, Liu H Z. Effect of samarium on methanation resistance of activated carbon supported ruthenium catalyst for ammonia synthesis[J]. J Rare Earths, 2010, 28(4): 552–555. DOI:10.1016/S1002-0721(09)60152-6 |
| [7] | Hara M, KitanoM , Hosono H. Ru-Loaded C12A7:e- Electride as a catalyst for ammonia synthesis[J]. ACS Catal, 2017, 7(4): 2313–2324. DOI:10.1021/acscatal.6b03357 |
| [8] | Abe H, Niwa Y, Kitano M, et al. Anchoring bond between Ru and N atoms of Ru/Ca2NH catalyst:Crucial for the high ammonia synthesis activity[J]. J Phy Chem C, 2017, 121(38): 20900–20904. DOI:10.1021/acs.jpcc.7b07268 |
| [9] | Jiang W, Li Y, Han W, et al. Effect of the graphitic degree of carbon supports on the catalytic performance of ammonia synthesis over Ba-Ru-K/HSGC catalyst[J]. J Ener Chem, 2014, 23(4): 443–452. DOI:10.1016/S2095-4956(14)60170-4 |
| [10] | Rarog-Pilecka W, Miskiewicz E, Szmigiel D, et al. Structure sensitivity of ammonia synthesis over promoted ruthenium catalysts supported on graphitised carbon[J]. J Catal, 2005, 231(1): 11–19. DOI:10.1016/j.jcat.2004.12.005 |
| [11] | Truszkiewicz E, Rarog-Pilecka W, Schmidt-Szatowski K, et al. Barium-promoted Ru/carbon catalyst for ammonia synthesis:State of the system when operating[J]. J Catal, 2009, 265(2): 181–190. DOI:10.1016/j.jcat.2009.04.024 |
| [12] | Wang Z, Ma Y, Lin J. Ruthenium catalyst supported on high-surface-area basic ZrO2 for ammonia synthesis[J]. J Mol Catal A:Chem, 2013, 378: 307–313. DOI:10.1016/j.molcata.2013.07.003 |
| [13] | Niwa Y, Aika K. Ruthenium catalyst supported on CeO2 for ammonia synthesis[J]. Chem Lett, 1996, 25(1): 3–4. |
| [14] | Lin J, Zhang L, Wang Z, et al. The effect of Ag as a promoter for Ru/CeO2 catalysts in ammonia synthesis[J]. J Mol Catal A:Chem, 2013, 366: 375–379. DOI:10.1016/j.molcata.2012.10.019 |
| [15] | Ma Z, Xiong X, Song C, et al. Electronic metal-support interactions enhance the ammonia synthesis activity over ruthenium supported on Zr-modified CeO2 catalysts[J]. RSC Adv, 2016, 6(56): 51106–51110. DOI:10.1039/C6RA10540H |
| [16] | Ma Z, Zhao S, Pei X, et al. New insights into the support morphology-dependent ammonia synthesis activity of Ru/CeO2 catalysts[J]. Catal Sci & Technol, 2017, 7(1): 191–199. |
| [17] | Lin B, Liu Y, Heng L, et al. Effect of ceria morphology on the catalytic activity of Co/CeO2 catalyst for ammonia synthesis[J]. Catal Commun, 2017, 101: 15–19. DOI:10.1016/j.catcom.2017.07.015 |
| [18] | Wang N, Qian W, Chu W, et al. Crystal-plane effect of nanoscale CeO2 on the catalytic performance of Ni/CeO2 catalysts for methane dry reforming[J]. Catal Sci & Technol, 2016, 6(10): 3594–3605. |
| [19] | Nolan M. Enhanced oxygen vacancy formation in ceria (111) and (110) surfaces doped with divalent cations[J]. J Mater Chem, 2011, 21(25): 9160–9168. DOI:10.1039/c1jm11238d |
| [20] | Xu Y, Greeley J, Mavrikakis M. Effect of subsurface oxygen on the reactivity of the Ag(111) surface[J]. J Am Chem Soc, 2005, 127(37): 12823–12827. DOI:10.1021/ja043727m |
| [21] | Bruix A, Rodriguez J A, Ramírez P J, et al. A new type of strong metal-support interaction and the production of H2 through the transformation of water on Pt/CeO2(111) and Pt/CeOx/TiO2(110) catalysts[J]. J Am Chem Soc, 2012, 134(21): 8968–8974. DOI:10.1021/ja302070k |
| [22] | Hu Z, Liu X, Meng D, et al. Effect of ceria crystal plane on the physicochemical and catalytic properties of Pd/Ceria for CO and propane oxidation[J]. ACS Catal, 2016, 6(4): 2265–2279. DOI:10.1021/acscatal.5b02617 |
| [23] | Dupin J C, Gonbeau D, Vinatier P, et al. Systematic XPS studies of metal oxides, hydroxides and peroxides[J]. Phys Chem Chem Phys, 2000, 2(6): 1319–1324. DOI:10.1039/a908800h |
| [24] | Piumetti M, Andana T, Bensaid S, et al. Ceria-based nanomaterials as catalysts for CO oxidation and soot combustion:Effect of Zr-Pr doping and structural properties on the catalytic activity[J]. AIChE J, 2017, 63(1): 216–225. DOI:10.1002/aic.v63.1 |
| [25] | Wang F, Li C, Zhang X, et al. Catalytic behavior of supported Ru nanoparticles on the {100}, {110}, and {111} facet of CeO2[J]. J Catal, 2015, 329: 177–186. DOI:10.1016/j.jcat.2015.05.014 |
| [26] | Gopiraman M, Bang H, Babu S G, et al. Catalytic N-oxidation of tertiary amines on RuO2 NPs anchored graphene nanoplatelets[J]. Catal Sci & Technol, 2014, 4(7): 2099–2106. |
| [27] | Huang H, Dai Q, Wang X. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene[J]. Appl Catal B:Environ, 2014, 158/159: 96–105. DOI:10.1016/j.apcatb.2014.01.062 |
| [28] | Walker A P, Rayment T, Lambert R M, et al. Structure and reactivity of ammonia synthesis catalysts derived from CeRu2 precursors:A study by in Situ X-ray absorption spectroscopy[J]. J Catal, 1990, 125(1): 67–76. DOI:10.1016/0021-9517(90)90078-X |
| [29] | Walker A P, Lambert R M. Properties of the ruthenium (0001)/cerium-hydrogen interface:A model system for transition-metal/rare-earth hydride catalysts[J]. The J Phy Chem, 1992, 96(5): 2265–2271. DOI:10.1021/j100184a044 |
| [30] | Ni Jun(倪军), Liu Ben-yao(刘本耀), Zhu Yong-long(朱本龙), et al. Effects of dual structure promoters on Ru/AC catalyst for ammonia synthesis(双结构助剂对Ru/AC氨合成催化剂稳定性的影响)[J]. J Mol Catal(China)(分子催化), 2013, 27(4): 371–376. |
| [31] | Yang Xiao-long(杨晓龙), Tang Li-ping(唐立平), Xia Chun-gu(夏春谷), et al. Effect of different supports on physical and chemical properties and catalytic activity over Ba-Ru/MgO ammonia synthesis catalysts(不同MgO担体对Ba-Ru/MgO氨合成催化剂物化性质和反应性能的影响)[J]. J Mol Catal(China)(分子催化), 2012, 26(1): 1–9. |
 2018, Vol. 32
2018, Vol. 32


