It is well accepted that the elements in the Earth are produced by nuclear reactions in the stars in universe and segregated in the Earth. In the mean time, some element atoms can convert into other atoms via radioactive decay, such as U to Pd, by which we know the age of our Earth. One element or its isotope can also be converted into another element or corresponding isotope by natural nuclear reaction[1-3]. For example, the radionuclides 14C in Earth's atmosphere is formed by constantly bombing by the high energy cosmic rays and the most of 40Ar comes from the decay of 40K in the air. The natural transmutation of radioactive elements (such as plutonium and uranium) can yield other radioactive elements or isotopes, some are dangerous and harmful to human beings due to their strong radioactive emission[4-6].
By bombing the target elements with high energy particle in accelerators or Tokamak, one element can be converted into another element, which is called artificial transmutation[2, 7]. The first artificial transmutation from N into O was done by Rutherford in 1919, who used α particle bomb nitrogen atoms to produce 17O, known as 14N + α → 17O + p[2, 4-6]. John Cockcroft and Ernest Walton fulfilled an artificial nuclear reaction by bombing7Li with accelerated protons to split the Li nucleus into two α particles in 1932, known as "splitting the atom"[8]. Otto Hahn et al. discovered that the artificial uranium fission in 1938[9-11]. From 1991 to 1997 in the Laboratori Nazionali del Gran Sasso, Ga (gallium trichloride-hydrochloric acid solution as the target) transmutation into Ge through a neutrino-induced nuclear reaction was reported, via reaction: νe + 71Ga → 71Ge + e-[12-13]. Although these transmutation processes require high energy and high-cost equipment, the artificial nuclear transmutation has been considered as a possible way to convert the radioactive waste to less hazardous nuclear elements via accelerator[4, 7].
Surprisingly, many experiments reported that element transmutation could be initiated under much lower energy level through metabolism processes of vegetal and animal organisms[14-21]. Some living organisms exhibit capability to transmute one element into another under very mild conditions. For example, Kervran et al. reported potassium and calcium contents changed during the growth of 840 seeds and 403 sprouts, their results indicated potassium might transmute into calcium during seeds growing, which could be represented in formula: 39K + 1p → 40Ca + △E[15-19]. Some other elemental transmutations were also reported, such as sodium to magnesium (23Na + n → 24Na* → 24Mg+e-+ ve*) and manganese to iron (55Mn + n → 56Mn* → 56Fe + e- + ve*)[20]. It was found that bio-transmutations were associated with ATP hydrolysis processes catalyzed by ATPase in biological bodies[21]. It is suggest that the elemental transmutation is essential to maintain a balance of certain elements in the biological bodies, which is critical to organism growth.
Most recently, we found such a low energy nuclear reaction could be achieved under normal conditions. Experimental results indicated that small amount of deuterium and helium can be produced during photocatalytic hydrogen evolution process from water catalyzed by Pt-graphene sensitized with Br-dye under visible light irradiation[3]. Similarly, helium 3 can be formed during photocatalytic hydrogen generation over CdS semiconductor dispersion under visible light irradiation[7]. In addition, we found potassium could be transmuted into calcium during photocatalytic hydrogen evolution in water mixture dispersion of dye (Eosin Y) and catalyst under irradiation of light with wavelength longer than 440 nm. We proposed that calcium atoms might originate from K-Ca transmutation through low energy nuclear reaction (LENR) under very mild conditions, which might be related to the involvement of negative hydrogen (hydride, H-) formed during photocatalytic hydrogen generation[2].
In fact, hydride, the negative hydrogen, can be provided from some compounds, such as NaBH4, LiBH4 and NH3BH3, in which, the H atoms attached to boron are hydride[2, 22]. If this hydride is offered in the mixture with potassium compound, the transmutation of potassium into calcium will take place. The electro-negativity (H-), hydrides, will initiate potassium transmutation.
In this work, we found the transmutation of K-Ca with negative hydrogen from NaBH4, LiBH4 and NH3BH3 in the mixture of K and those chemicals. Here, K+ was chosen as the target element, and K+ and Ca2+ concentrations in the mixture were monitored by inductive coupled plasma optical emission spectroscopy (ICP-OES) and inductive coupled plasma mass spectrometry (ICP-MS) techniques. The results showed K+ concentration was gradually decreasing, and the Ca2+ concentration was gradually increasing. By comparing the K and Ca concentrations and their isotopes, we found K to Ca transmutation taking place in the presence of hydride, while the increasing concentration of 41K correlating to the increase of 40Ca concentration under our "reaction" conditions, which implying 40Ca formation correlated to 41K.
1 Experimental details 1.1 Chemicals and MaterialsAll chemicals were purchased and used without further purification.NaBH4 (Shanghai Guangming Chemical Reagent Co., Ltd, AR, ≥ 97%), LiBH4 (Sam Chemical Technology Co., Ltd, AR, ≥ 95%), NH3BH3 (Sinopharm Chemical Reagent Co., Ltd, AR, ≥ 90%), KCl (Xilong Chemical Co., Ltd., AR, ≥ 99.5%), HCl (BeiJing Chemical Works, GR, 36%~38%), Ca(NO3)2 (Xilong Chemical Co., Ltd., AR, ≥ 99.5%) and RuCl3 (Shanghai Guangming Chemical Reagent Co., Ltd, AR, ≥ 37.3%). Ultrapure water: 18.2 MΩ·cm-1 at 25 ℃ (Milli-Q water, Millipore Mili-Q reference ultrapure water purification system, USA) was used in this study.
Since the polypropylene (PP) volumetric flask only has two elements (C and O), it was chemical stable in the acidic and alkaline environment. Therefore, the involvment of other elements can be ignored from the PP volumetric flask, which was used as a reactor and a fixed vessel for all experiments. Prior to the reaction, all PP volumetric flasks (100 mL) were recalibrated and washed several times with high pure Mill-Q water, and then they were dried at room temperature.
1.2 Element transmutation experimental 1.2.1 NaBH4+KCl systemThe element transmutation was performed at room temperature in a 100 mL PP volumetric flask. 1.0 mL of KCl solution (CK=170 ppm) was placed into the PP volumetric flask with 50 mL Mill-Q water, which was uniformly dispersed by ultrasound treatment 5 min. Then, the calculated amount of negative hydrogen chemical NaBH4 (945.8 mg) was added in the aqueous solution of KCl by adjusting reaction temperature and time. In order to eliminate the test error caused by the precipitation of calcium in the alkaline environment, the pH of reaction solution was adjusted by adding 1 mL HCl. After that, the mixed solution was titrated to 100 mL with Mill-Q high purity water and was kept over the designed reaction time.
1.2.2 LiBH4+KCl systemIn order to further prove potential transmutation from potassium to calcium via hydride H- via low energy nuclear reaction, NaBH4 was substituted with another negative hydrogen chemical LiBH4. In the LiBH4+KCl system, the experimental process and conditions were similar to the NaBH4+KCl system.
1.2.3 NH3BH3+KCl+RuCl3+Ca(NO3)2 system50.0 mL of Ca(NO3)2 solution (CCa=100 ppm), 1.0 mL of KCl solution (CK=170 ppm), and 2.0 mL RuCl3 solution (20 mmol/L) were added in a 100 mL PP volumetric flask. After being ultrasound treatment for 5 min, NH3BH3 of different content was added in the mixture solution for 2 days until no bubbles were generated under the room temperature. Similarly, the pH of reaction solution was adjusted by adding 1 mL HCl. And the mixed solution was also titrated to 100 mL with Mill-Q high purity water.
1.3 Measurements and AnalysisTo verify the element transmutation in the presence of hydride, the negative hydrogen, inductive coupled plasma optical emission spectroscopy (ICP-OES, 730) and inductive coupled plasma mass spectrometry (ICP-MS, 7700) measurements were conducted. ICP-MS was mainly used to detect changes in low concentration elements and isotopes. The samples after reaction were directly extracted and detected by the ICP-OES and ICP-MS, and all the solutions tested were not further diluted prior to analysis. The every sample was detected at least three times and the average value was presented.
2 Results and discussionICP-OES measurement is conducted to monitor the concentration variation of elements in the reaction mixture to verify the element transmutation in the presence of hydride, and the corresponding results are given in Fig. 1a. For the NaBH4 case, the Ca2+ concentrations in the NaBH4+KCl mixture are 0.131±0.010, 0.069±0.008, 0.258±0.012 and 0.427±0.013 ppm, under the reaction temperature after 2 h reaction at 0, 25, 50 and 75 ℃, respectively. Distinctly, the Ca2+ concentration of the NaBH4+KCl system gradually increase and are strongly dependent on the reaction temperature. It needs to be pointed out that there is no additional calcium source in these initial reaction mixtures. The ICP-OES measurements for every datum are repeated for three times and the average value is presented. Fig. 1a also displays the change of K+ concentration with the increase of reaction temperature. The concentration of K decreased with the reaction operation.
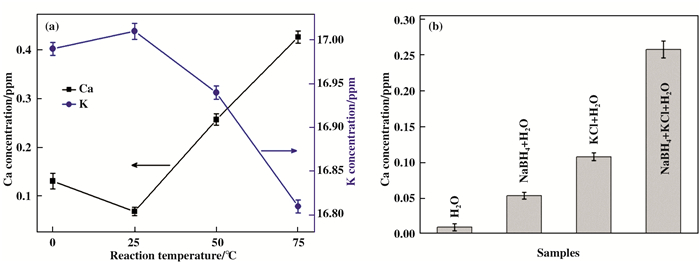
|
Fig.1 The elements concentrationare detected by ICP-OES. (a) Ca2+ and K+ concentration of NaBH4+KCl system at 0, 25, 50 and 75 ℃ after reaction 2 h. 100 mL aqueous solution of KCl (4.115×10-5 mol) and NaBH4 (0.025 mol); (b) Ca2+ concentration of the H2O, NaBH4, KCl, and NaBH4+KCl system at 50 ℃ after reaction 2 h. |
To avoid misunderstanding of the results, we double checked the initial Ca concentration in Milli-Q H2O, NaBH4 and KCl solutions by ICP-OES (pre-heated to 50 ℃ and kept pH=7). As shown in Fig. 1b, the Ca2+ concentration in Milli-Q H2O is only 0.010±0.005 ppm. For the NaBH4 and KCl aqueous solution, the Ca2+ concentrations are 0.053±0.005 and 0.108±0.006 ppm, respectively. However, for the NaBH4+KCl reaction mixture, the Ca2+ concentration obviously increase to 0.258±0.012 ppm. It is doubtless that the excess Ca in the mixture originated from the reaction of NaBH4 and KCl.
To further study the K-Ca transmutation reaction, the cyclic experiments was done and the results were presented in Fig. 2. The concentration of Ca2+ ions increases continuously when fresh NaBH4 is introduced in the reaction mixture. After four cycles of NaBH4 addition, the concentration of Ca2+ reached 0.356±0.008 ppm. The ICP-OES data obvious confirmed that the concentration of Ca2+ increases with the reaction time and the amount of NaBH4 in mixture. The final Ca2+ concentration increased up to 0.356 ppm after the four cycles. Although there is fluctuation of measured Ca concentration, the increase tendency of calcium is very obvious during reaction.
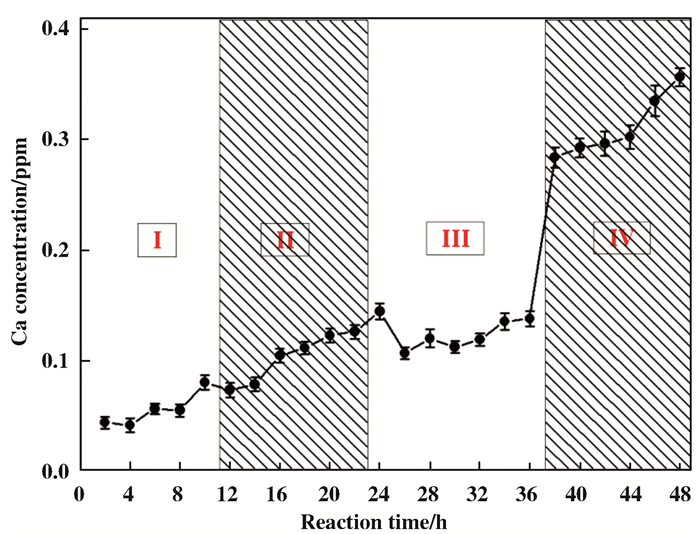
|
Fig.2 Cyclic experiment of NaBH4+KCl system at 25 ℃, (Ⅰ) First cycle, [NaBH4]: 0. 025 mol; [K+]: 4.115× 10-5 mol; (Ⅱ) addition of fresh NaBH4 (0.025 mol); (Ⅲ) third repeat; (Ⅳ) fourth repeat. |
These are exotic phenomena that the increase of calcium concentration in NaBH4+KCl reaction mixture. Given that the reaction system is conducted in well sealed container which could prevent any exogenous contaminate, there is almost no choice but to believe that some substance in the reactive mixture might be converted to Ca element. Considering previous bio-transmutation research mentioned in introduction section (39K + 1p = 40Ca + △E) and based on the above experimental results, a feasible way is the potassium element transmutation into calcium via H- LENR in the NaBH4+KCl system under a mild condition[15-19].
In order to further prove above suggestion of this potential transmutation from potassium to calcium via H- LENR, comparative experiments are carried out by substituting NaBH4 with LiBH4 in the reaction mixture. Comparative experiments are repeated for several times, and their ICP-OES analysis results are presented in Fig. 3. The results indicated the concentration of Ca2+ ions increased continuously in the LiBH4+KCl system. Correspondingly, the content of potassium decrease in the same system. Those results provide evidence that the increase of calcium concentration in the reaction mixture is closely related to the presence of H-, namely, indicating elemental transmutation from potassium to calcium.
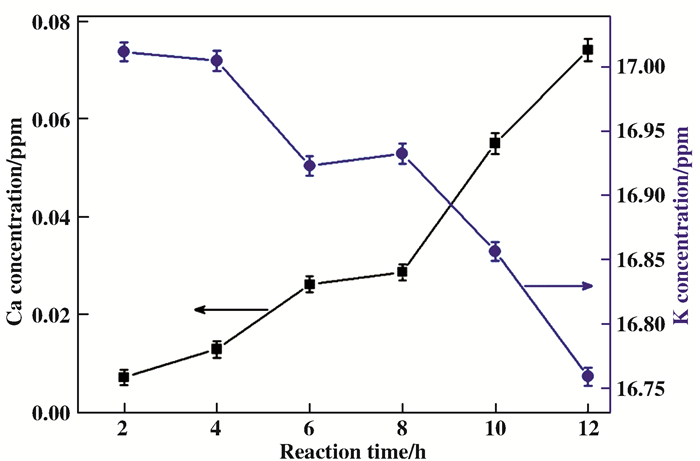
|
Fig.3 Ca2+ and K+ concentration of LiBH4+KCl system at various time are detected by ICP-OES at 0 ℃ after reaction 2 h. 100 mL aqueous solution of KCl (4.115×10-5 mol) and LiBH4 (0.025 mol). |
Moreover, we use ICP-MS to detect the change of corresponding elemental isotope in the NH3BH3+KCl+RuCl3+Ca(NO3)2 mixture. To verify the accuracy and credibility of ICP-MS results, other elements and their isotopes were simultaneously measured, such as Mo, becasuse there was no Mo in the mixture. Mo, here, was used as a proble element to check if the reaction was contaminated. Fig. 4a shows the ICP-MS results of the measured concetntrations of Mo isotope in the different NH3BH3 loading (1#, 358.5 mg; 2#, 717.0 mg; 3#, 1075.5 mg), as well as in other used chemical reagents, KCl, RuCl3 and Ca(NO3)2. The results indicated that the isotopes content of Mo are very low, and the ratio between different isotopes of Mo remains almost unchanged in the reaction mixture (Fig. 4b). This mean that the ICP-MS can be used to detect and track the minor change of elemental isotopes.
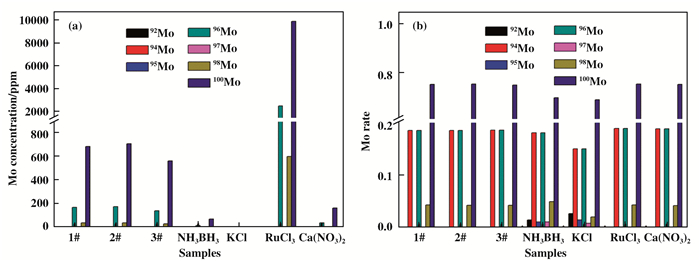
|
Fig.4 (a) Mo isotope concentration of NH3BH3+KCl+RuCl3+Ca(NO3)2 system with different NH3BH content 1# (358.5 mg), 2# (717.0 mg) and 3# (1075.5 mg), and the isotope content in NH3BH3, KCl, RuCl3 and Ca(NO3)2 are detected by ICP-MS. (b) the corresponding isotope ratio in their respective systems. |
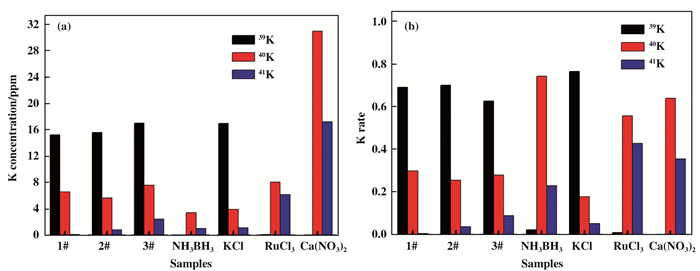
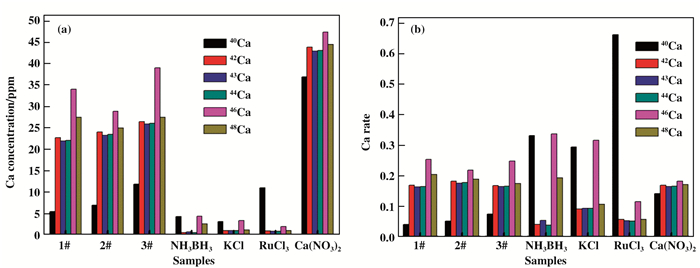
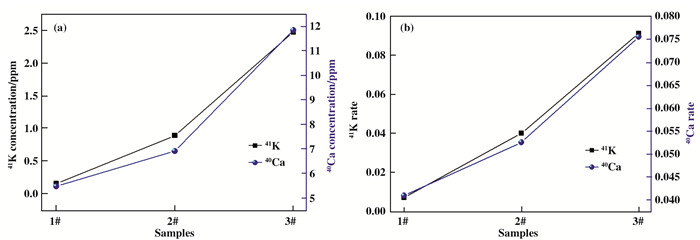
Fig. 5a and Fig. 6a showthe ICP-MS results of K and Ca isotope concentration in NH3BH3+KCl+RuCl3+Ca(NO3)2 mixture with different NH3BH3 loading. We can find that the K isotope ratio, such as 39K, 40K and 41K, have obvious changes after the raection, as shown in Fig. 5b. In particular, the amount of isotope 41K gradually increase with the increase of negative hydrogen loading from NH3BH3. In additon, we can clearly see that the isotopes of Ca exhibits similar changes in Fig. 6b. Compared with isotope 42Ca, 43Ca, 44Ca, 46Ca and 48Ca, the amount of isotope 40Ca also increase with the increase of NH3BH3. The variation of 41K and 40Ca is obviously not due to the experimental error, as shown in Fig. 7a and 7b.
|
Fig.5 (a) K isotope concentration of NH3BH3+KCl+RuCl3+Ca(NO3)2 system with different NH3BH content 1# (358.5 mg), 2# (717.0 mg) and 3# (1075.5 mg), and the isotope content in NH3BH3, KCl, RuCl3 and Ca(NO3)2 are detected by ICP-MS. (b) the corresponding K isotope ratio. |
|
Fig.6 (a) Ca isotope concentration of NH3BH3+KCl+RuCl3+Ca(NO3)2 system with different NH3BH content 1# (358.5 mg), 2# (717.0 mg) and 3# (1075.5 mg), and the isotope content in NH3BH3, KCl, RuCl3 and Ca(NO3)2 are detected by ICP-MS. (b) the corresponding Ca isotope ratio. |
|
Fig.7 (a) 41K and 40Ca isotope concentration of NH3BH3+KCl+RuCl3+Ca(NO3)2 system with different NH3BH content 1# (358.5 mg), 2# (717.0 mg) and 3# (1075.5 mg) are detected by ICP-MS. (b) the corresponding 41K and 40Ca isotope ratio. |
These surprising experimental results are difficult to interpret and even beyond our existing theoretical knowledge. However, based on the above experimental results, a plausible transmutation process might be shown in Scheme 1. Negative hydrogen compound molecule (such as NaBH4) is easy to hydrolyze and produce H- ions, which is a complex substance composed of a hydrogen atom that weakly binds two electrons and can be seen as a baryon[23]. Due to the strong electrostatic attraction, K+ ions that come from KCl could randomly capture a H- in a manner we still not clearly understand, probably by nucleus-electron-nucleus interaction, forming H- baryon-potassium intermediate, and this intermediate(s) could decay to 41K and 40Ca:
| $ \text{NaB}{{\text{H}}_{4}}~+\text{ }2{{\text{H}}_{2}}\text{O }\leftrightarrow \text{ N}{{\text{a}}^{+}}~+\text{ B}{{\text{O}}_{2}}{{^{~}}^{-}}\text{ }+\text{ }4{{\text{H}}^{+}}~+\text{ }4{{\text{H}}^{-}} $ | (1) |
| $ ^{39}{{\text{K}}^{+}}~+{{~}^{1}}{{\text{H}}^{-}}\to {{~}^{40}}\text{C}{{\text{a}}^{2+}}~+\text{ }2{{\text{e}}^{-}}~\Delta \text{E} $ | (2) |

|
Scheme 1 Scheme diagram of a typical K-Ca transmutation process |
Transmutation process could mainly be attributed to the formation of H--K intermediate[22]. The H- baryon, like electrons in ordinary atoms, approaching the potassium nucleus. According to the Bohr Theory, the atomic orbital radius (rn) is inversely proportional to the mass of H- baryon:
| $ \begin{align} &{{r}_{n}}\approx \frac{{{h}^{2}}}{8{{\pi }^{2}}z{{e}^{2}}m}\left[3{{n}^{2}}-l\left( l+1 \right) \right] \\ &\ \ \ \approx \frac{{{m}_{e}}}{m}\frac{\left[3{{n}^{2}}-l\left( l+1 \right) \right]}{2z}{{a}_{0}} \\ \end{align} $ | (3) |
where h is Plank constant (6.6262×10-34 J s), n is main quantum number, l is angular quantum number, z is atomic number, e is charge of negative ions (1.6022×10-19 C), me is electronic mass (9.1096×10-31 kg), m is the mass of negative ions in the atom (1.6811×10-27 kg), a0 is the Bohr radius of H atom (0.5292×10-10 m)[24-25]. For the H--K, if the outer valence electron of K is occupied by H- baryon, and the possible particle radius r is about 3.5216×10-14 m, which is only 1/1836 of the normal K atom. This means that the radius of H--K is in the same order of magnitude as the radius of the nucleus. So that the H- baryon has a higher probability to enter the K nucleus.
The energy levels of H--K have also changed greatly. From the formula (4), we find that the H- baryon does not move on the original electron orbit, but has its own set of energy level orbits. These orbital energy levels (En) can be determined by the following formula:
| $ {{E}_{n}}~=-\frac{m{{c}^{2}}}{2}{{\left( \frac{\alpha z}{n} \right)}^{2}}\left[1+{{\left( \frac{\alpha z}{n} \right)}^{2}}\left( \frac{n}{j+\frac{1}{2}}-\frac{3}{4} \right)-\cdots \right] $ | (4) |
where c and α are the light speed and fine-structure constant; j is total angular momentum quantum number of negative particles. The meaning of other symbols is the same as that of the former formula. For this particular structure, En depends on the H- baryon mass. Compared to the normal electronic transitions, H- baryon released energy is higher.
As for the mass difference before and after reaction in formula (4), it should corresponds some energy variation during this reaction. According to the mass-energy equation, E = mc2, the mass difference may correlate energy releasing or new matter formation, for example, some quarks.
| $ {{\text{K}}^{+}}+\text{ }{{\text{H}}^{-}}\to \text{C}{{\text{a}}^{2+}}+\text{ }2{{\text{e}}^{-}}+\Delta \text{m }({{\text{s}}^{-1/3}}+\text{ }{{\text{u}}^{+2/3}}+{{\text{d}}^{-1/3}}) $ | (5) |
A lot of research facts show that calcium is an important element for human body, like constituting the bones and teeth and helping heart and other muscles do their work normally[26-30]. Low calcium intake could lead to fragile bones, high blood pressure, and certain types of cancer. Actually, H- can act as antioxidant by selectively reducing cytotoxic oxygen radicals[30]. It could be supposed that there might be hydrogen which existing as negative hydrogen in human body, for H- ions are more solvable in body fluids compared with H2 molecule. Those H- ions could facilitate elements transmutation in organism body. Base on the reported data and proposed mechanism, we can suppose that K-Ca transmutation is beneficial for maintaining human body health, which might be a potential answer to a long-lastingly unsettled question, that what environment can induce "mild" element transmutation, for example, the transmutation in biological bodies and bionics reactive system, typically, energy release and new matter formation via such a LENR. It can also help to answer the question that why the chickens can produce so many eggs while they do not take in enough calcium, and how can organisms take in some special elements even they grow in isolated environments. In addition, the mitochondrion in cells might capture the energy released from transmutation, accumulate those energies, and release them slowly for organism metabolism.
3 ConclusionWe finds some exotic and interesting results about potassium transmutation to calcium in the mixture containing K+ and H-. The results showed K+ concentration is gradually decreasing, while the Ca2+ concentration is gradually increasing in the mixture dispersion. Those results indicate that some of calcium elements in nature might originate from K-Ca transmutation through LENR under very mild conditions, which might be related to the yield of H-. Besides, we can make some predictions if the formation of K-Ca transmutation follows the mechanism of the combination of K+ and H- baryon. One can achieve the element transmutation of some light nucleus to a little bit heavy nucleus base on the H- baryon, such as transmutation from Na to Mg, Mn to Fe, or Cs to Ba under mild conditions. These reactions can help us further understand the Ca formation and loss mechanism in our body and remove dangerous nuclear waste like 137Cs. We realize those discoveries may raise some questions and arguments.
Acknowledgements:
This work is supported by the National Natural Science Foundation of China (Grant Nos. 21673262 and 21433007), respectively.
| [1] | Schaeffer O A, Zähringer J. Solar flare helium in satellite materials[J]. Phys Rev Lett, 1962, 8(10): 389. DOI:10.1103/PhysRevLett.8.389 |
| [2] | Lu Gong-xuan(吕功煊), Zhang Wen-yan(张文妍). Photocatalytic hydrogen evolutionand induced transmutation of potassium to calcium via low-energy nuclear reaction (LENR) driven by visible light(可见光驱动的光催化产氢同时诱导低能核反应嬗变钾为钙)[J]. J Mol Catal(分子催化), 2017, 31(5): 401–410. |
| [3] | Lu Gong-xuan(吕功煊), Tian Bin(田彬). Formation of deuterium and helium during photocatalytic hydrogen generation from water catalyzed by Pt-graphene sensitized with Br-dye under visible light irradiation(溴染料敏化担载Pt石墨烯催化可见光制氢、氘和氦)[J]. J Mol Catal(分子催化), 2017, 31(2): 101–104. |
| [4] | Badash L. Radium, Radioactivity, and the popularity of scientific discovery[J]. Proc Am Philos Soc, 1978, 122(3): 145–154. |
| [5] | Howorth M. Pioneer research on the atom:Rutherford and soddy in a glorious chapter of science; the life story of Frederick Soddy[M]. New World Publications, 1958. |
| [6] | Trenn T J. The self-splitting atom:the history of the rutherford-soddy collaboration[J]. London:Taylor & Francis, 1977, 42(58/60): 111–117. |
| [7] | Lu Gong-xuan(吕功煊), Zhen Wen-long(甄文龙). Formation of deuterium and helium during photocatalytic hydrogen generation from water catalyzed by Pt-graphene sensitized with Br-dye under visible light irradiation(半导体CdS悬浮体系中可见光催化产氢同时生成氦-3和氦-4)[J]. J Mol Catal(分子催化), 2017, 31(4): 299–304. |
| [8] | Cockcroft J D, Walton E T S. Artificial production of fast protons (Reprinted from Nature, February 13, 1932)[J]. Nature, 1969, 224: 463. DOI:10.1038/224463a0 |
| [9] | Henderson M C. The disintegration of lithium by protons of high energy[J]. Phys Rev, 1933, 43(2): 98–102. |
| [10] | Paneth F A, Günyher P L. Chemical detection of artificial transmutation of elements[J]. Nature, 1933, 131: 652–653. |
| [11] | Bush R P. Recovery of platinum group metals from high level radioactive waste[J]. Platinum Metals Rev, 1991, 35(4): 202–208. |
| [12] | Guerra F., MLeone M. Robotti N, The discovery of artificial radioactivity[J]. Phys Pers, 2012, 14(1): 33–58. |
| [13] | Davis J R, Harmer D S, Hoffman K C. Search for neutrinos from the sun[J]. Phys Rev Lett, 1968, 20(21): 1205. DOI:10.1103/PhysRevLett.20.1205 |
| [14] | Abazov A I, Anosov O L, Faizov E L, et al. Search for neutrinos from the Sun using the reaction Ga 71Ga(νe, e-)71 Ge[J]. Phys Rev Lett, 1991, 67(24): 3332. DOI:10.1103/PhysRevLett.67.3332 |
| [15] | Kervran C L. Biological transmutations and modern physics[J]. Publi Malo SA, Paris, 1982. |
| [16] | Biberian J P. Biological transmutations:Historical perspective[J]. J Cond Matter Nucl Sci, 2012, 7: 11–15. |
| [17] | Biberian J P. Biological transmutations[J]. Curr Sci, 2015, 108(4): 633–635. |
| [18] | Kozima H, Hiroe K, Nomura M, et al. On the elemental transmutation in biological and chemical systems[J]. Cold Fusion, 1996, 16: 30. |
| [19] | Vysotskii V I, Adamenko S V, Vysotskyy M V. Acceleration of low energy nuclear reactions by formation of correlated states of interacting particles in dynamical systems[J]. Ann Nucl Energy, 2013, 62: 618–625. DOI:10.1016/j.anucene.2013.02.021 |
| [20] | Moawad E Y. Nuclear transmutation and cancer in the biological cell[J]. Int J Biochem Biophys, 2013, 1(1): 1–8. |
| [21] | Timashev S F. Initiating nuclear-chemical transformations in native systems:Phenomenology[J]. Russ J Phys Chem A, 2016, 90(10): 2089–2095. DOI:10.1134/S0036024416100253 |
| [22] | Yukawa H, Sakata S, Taketani M. On the interaction of elementary particles. Ⅲ[J]. Proce Phys-Mathe Soc Japan 3rd Series, 1938, 20: 319–340. |
| [23] | Zhang X Q, Wang C W, Chen J B, et al. Enhancement of the field emission from the TiO2 nanotube arrays by reducing in a NaBH4 solution[J]. ACS Appl Mater Interf, 2014, 6(23): 20625–20633. DOI:10.1021/am503379y |
| [24] | Fano U. Penetration of protons, alpha particles, and mesons[J]. Annu Rev Nucl Sci, 1963, 13(1): 1–66. DOI:10.1146/annurev.ns.13.120163.000245 |
| [25] | Afanasyev L G, Chvyrov A S, Gorchakov O E., et al. Observations of atoms consisting of π+ and π- mesons[J]. Phys Lett B, 1993, 308(1/2): 200–206. |
| [26] | Chen Y, Gu W J, Pan H H, et al. Stabilizing amorphous calcium phosphate phase by citrate adsorption[J]. CrystEngComm, 2014, 16(10): 1864–1867. DOI:10.1039/C3CE42274G |
| [27] | Qiu X C, Zhang Y S, Zhu Y Q. Spectrophotometric determination of exchangeable calcium in soils by chlorophosphonazo-mA[J]. Analyst, 1983, 108(1287): 754–757. DOI:10.1039/an9830800754 |
| [28] | Pravina P, Sayaji D, Avinash M. Calcium and its role in human body[J]. Int J Res Pharm Biomed Sci, 2013, 4(2): 659–668. |
| [29] | Dougherty D P, Wright G A, Yew A C. Computational model of the cAMP-mediated sensory response and calcium-dependent adaptation in vertebrate olfactory receptor neurons[J]. Proc National Acad Sci, 2005, 102(30): 10415–10420. DOI:10.1073/pnas.0504099102 |
| [30] | Ohsawa I, Ishikawa M, Takahashi K, et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals[J]. Nat Med, 2007, 13(6): 688–694. DOI:10.1038/nm1577 |
 2019, Vol. 33
2019, Vol. 33

